Aconitum pseudo-laeve var. erectum Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclastogenesis via the c-Fos/nuclear Factor of Activated T-Cells, Cytoplasmic 1 Signaling Pathway and Prevents Lipopolysaccharide-Induced Bone Loss in Mice
Abstract
:1. Introduction
2. Results and Discussion
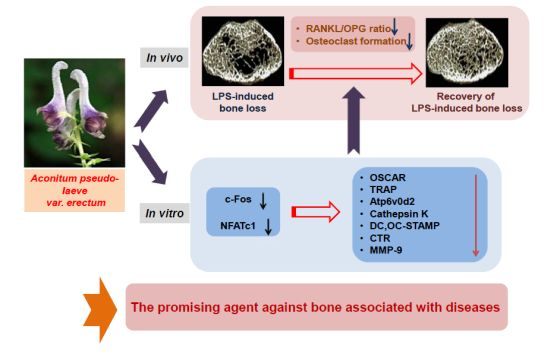
2.1. Administration of APE Restores LPS-Induced Bone Loss in Vivo
2.2. APE Restores LPS-Induced Bone Loss by Inhibiting the RANKL/OPG Ratio and Osteoclast Formation


2.3. APE Prevents the Formation of TRAP-positive Osteoclasts and the Bone Resorbing Ability of Mature Osteoclasts

2.4. The Inhibitory Effect of APE on c-Fos and NFATc1 Activation Is Not Associated with Early Signal Pathways
2.5. APE Regulates Osteoclastogenesis through Suppressing the mRNA Expression of Osteoclast Marker Genes


3. Experimental Section
3.1. Reagents and Antibodies
3.2. Mouse Bone Marrow Cell (BMC) Isolation and Osteoclast Differentiation
3.3. Cell Viability Assay
3.4. Western Blot Analysis
3.5. Quantitative Real-time PCR Analysis
| Primer sequences for Real-time RT-PCR | |
|---|---|
| c-Fos | Forward: 5'-GGT GAA GAC CGT GTC AGG AG-3' |
| NFATc1 | Forward: 5'-GAG TAC ACC TTC CAG CAC CTT-3' |
| TRAP | Forward: 5'-ACT TCC CCA GCC CTT ACT AC-3' |
| OSCAR | Forward: 5'-GGA ATG GTC CTC ATC TGC TT-3' |
| Cathepsin K | Forward: 5'-CCA GTG GGA GCT ATG GAA GA-3' |
| Atp6v0d2 | Forward: 5'-GAC CCT GTG GCA CTT TTT GT-3' |
| DC-STAMP | Forward: 5'-TCC TCC ATG AAC AAA CAG TTC CA-3' |
| OC-STAMP | Forward: 5'-ATG AGG ACC ATC AGG GCA GCC ACG-3' |
| Calcitonin receptor (CTR) | Forward: 5'-TCC AAC AAG GTG CTT GGG AA-3' |
| MMP-9 | Forward: 5'-TCC AAC CTC ACG GAC ACC C-3' |
| GAPDH | Forward: 5'-TCA AGA AGG TGG TGA AGC AG-3' |
3.6. Bone Resorption Assay
3.8. Mouse Model of LPS-induced Bone Erosion and Micro-CT and Histological Analysis
3.9. Measurement of RANKL and OPG
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.S.; Jiang, X.; Kagan, R.; Schnatz, P. Osteoporosis management in post-menopausal women. Minerva Ginecol. 2012, 64, 181–194. [Google Scholar] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends. Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, J.; Takaesu, G.; Akatsuka, H.; Sakurai, H.; Ninomiya-Tsuji, J.; Matsumoto, K.; Sakurai, N. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol. Cell. Biol. 2002, 22, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Gingery, A.; Bradley, E.; Shaw, A.; Oursler, M.J. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J. Cell Biochem. 2003, 89, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Epple, H.; Uthgenannt, B.; Novack, D.V.; Faccio, R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Invest. 2006, 116, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Kim, J.H.; Kim, K.; Jin, H.M.; Youn, B.U.; Kim, N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009, 583, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Suzuki, T.; Miyauchi, Y.; Iwasaki, R.; Kobayashi, T.; Sato, Y.; Miyamoto, K.; Hoshi, H.; Hashimoto, K.; Yoshida, S.; et al. Osteoclast stimulatory transmembrane protein and dendritic cell-specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 2012, 27, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Mizoguchi, T.; Harada, S.; Kobayashi, Y.; Nakamichi, Y.; Yasuda, H.; Penninger, J.M.; Yamada, K.; Udagawa, N.; Takahashi, N. Fos plays an essential role in the upregulation of RANK expression in osteoclast precursors within the bone microenvironment. J. Cell Sci. 2012, 125, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Ko, C.H.; Yue, G.G.; Lee, J.K.; Li, K.K.; Lee, M.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B. Green tea (Camellia sinensis) extract inhibits both the metastasis and osteolytic components of mammary cancer 4T1 lesions in mice. J. Nutr. Biochem. 2014, 25, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.S.; Zong, X.H.; Li, X.G. Controlled clinical trials of therapeutic effects of Chinese herbs promoting blood circulation and removing blood stasis. Zhongguo Gu Shang 2009, 22, 920–922. [Google Scholar] [PubMed]
- Jia, N.; Li, Y.; Wu, Y.; Xi, M.; Hur, G.; Zhang, X.; Cui, J.; Sun, W.; Wen, A. Comparison of the anti-inflammatory and analgesic effects of Gentiana macrophylla Pall. and Gentiana straminea Maxim., and identification of their active constituents. J. Ethnopharmacol. 2012, 144, 638–645. [Google Scholar]
- Hausmann, E.; Raisz, L.G.; Miller, W.A. Endotoxin: stimulation of bone resorption in tissue culture. Science 1970, 168, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Orcel, P.; Feuga, M.; Bielakoff, J.; De Vernejoul, M.C. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am. J. Physiol. 1993, 264, 391–397. [Google Scholar]
- Ikeda, T.; Kasai, M.; Utsuyama, M.; Hirokawa, K. Determination of three isoforms of the receptor activator of nuclear factor-[kappa]B ligand and their differential expression in bone and thymus. Endocrinology 2001, 142, 1419–1426. [Google Scholar] [PubMed]
- Khosla, S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoids-induced osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [PubMed]
- Findlay, D.M.; Atkins, G.J. Relationship between serum RANKL and RANKL in bone. Osteoporosis Int. 2011, 22, 2597–2602. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Kobayashi, K.; Jimi, E.; Udagawa, N.; Takahashi, N. The molecular basis of osteoclast differentiation and activation. Novartis Found. Symp. 2001, 232, 235–247. [Google Scholar] [PubMed]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Luxenburg, C.; Geblinger, D.; Klein, E.; Anderson, K.; Hanein, D.; Geiger, B.; Addadi, L. The architecture of the adhesive apparatus of cultured osteoclasts: From podosome formation to sealing zone assembly. PLoS One 2007, 2, e179. [Google Scholar] [CrossRef] [PubMed]
- Stenbeck, G. Formation and function of the ruffled border in osteoclasts. Semin. Cell Dev. Biol. 2002, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.K.; Horton, M. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J. Cell Sci. 1995, 108, 2729–2732. [Google Scholar] [PubMed]
- Nakamura, I.; Pilkington, M.F.; Lakkakorpi, P.T.; Lipfert, L.; Sims, S.M.; Dixon, S.J.; Rodan, G.A.; Duong, L.T. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999, 112, 3985–3993. [Google Scholar] [PubMed]
- Mulari, M.T.; Zhao, H.; Lakkakorpi, P.T.; Vaananen, H.K. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic 2003, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, P.H.; Blair, H.C.; Teitelbaum, S.L.; Edwards, J.C. Characterization of the osteoclast ruffled border chloride channel and its role in bone resorption. J. Biol. Chem. 1997, 272, 18636–18643. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Matsuo, K. Signalling in osteoclasts and the role of Fos/AP1 proteins. Ann. Rheum. Dis. 2003, 62, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Macian, F.; Lopez-Rodriguez, C.; Rao, A. Partners in transcription: NFAT and AP-1. Oncogene 2001, 20, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Cremasco, V.; Decker, C.E.; Stumpo, D.; Blackshear, P.J.; Nakayama, K.I.; Nakayama, K.; Lupu, T.S.; Graham, D.B.; Novack, D.V.; Faccio, R. Protein kinase C-delta deficiency perturbs bone homeostasis by selective uncoupling of cathepsin K secretion and ruffled border formation in osteoclasts. J. Bone Miner. Res. 2012, 27, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Stroup, G.B.; Lark, M.W.; Veber, D.F.; Bhattacharyya, A.; Blake, S.; Dare, L.C.; Erhard, K.F.; Hoffman, S.J.; James, I.E.; Marquis, R.W.; et al. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J. Bone Miner. Res. 2001, 16, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of APE is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baek, J.M.; Kim, J.-Y.; Cheon, Y.-H.; Park, S.-H.; Ahn, S.-J.; Yoon, K.-H.; Oh, J.; Lee, M.S. Aconitum pseudo-laeve var. erectum Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclastogenesis via the c-Fos/nuclear Factor of Activated T-Cells, Cytoplasmic 1 Signaling Pathway and Prevents Lipopolysaccharide-Induced Bone Loss in Mice. Molecules 2014, 19, 11628-11644. https://doi.org/10.3390/molecules190811628
Baek JM, Kim J-Y, Cheon Y-H, Park S-H, Ahn S-J, Yoon K-H, Oh J, Lee MS. Aconitum pseudo-laeve var. erectum Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclastogenesis via the c-Fos/nuclear Factor of Activated T-Cells, Cytoplasmic 1 Signaling Pathway and Prevents Lipopolysaccharide-Induced Bone Loss in Mice. Molecules. 2014; 19(8):11628-11644. https://doi.org/10.3390/molecules190811628
Chicago/Turabian StyleBaek, Jong Min, Ju-Young Kim, Yoon-Hee Cheon, Sun-Hyang Park, Sung-Jun Ahn, Kwon-Ha Yoon, Jaemin Oh, and Myeung Su Lee. 2014. "Aconitum pseudo-laeve var. erectum Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclastogenesis via the c-Fos/nuclear Factor of Activated T-Cells, Cytoplasmic 1 Signaling Pathway and Prevents Lipopolysaccharide-Induced Bone Loss in Mice" Molecules 19, no. 8: 11628-11644. https://doi.org/10.3390/molecules190811628