Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of tHGA Analogs


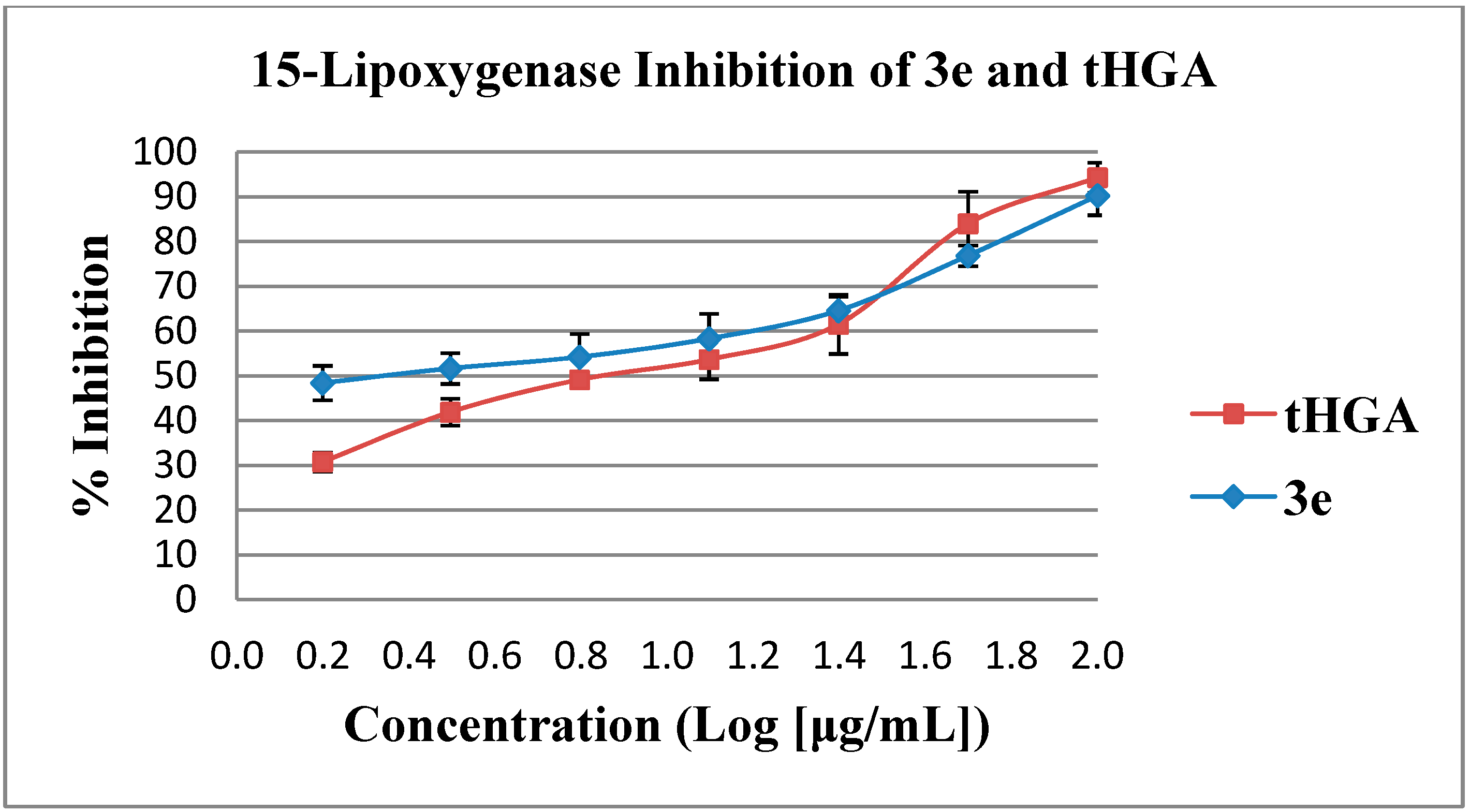
2.2. In Vitro Soybean 15-Lipoxygenase Inhibition Assay
| Compound | % Inhibition (100 μg/mL) | IC50 Value (μM) Mean ± SEM |
|---|---|---|
| 1 | 23.2 ± 2.2 | >100 |
| 2a | 19.4 ± 5.0 | >100 |
| 2c * | n.a | >100 |
| 2d | 16.9 ± 1.9 | >100 |
| 2e | 24.5 ± 3.2 | >100 |
| 2f | 17.5 ± 3.0 | >100 |
| 2g * | n.a | >100 |
| 3a Ω | 90.3 ± 4.1 | 27.61 ± 3.6 |
| 3c | 94.4 ± 3.0 | 12.32 ± 0.6 |
| 3e # | 90.3 ± 4.4 | 10.31 ± 1.5 |
| 3f | 88.8 ± 5.9 | 26.31 ± 1.3 |
| 3g | 90.8 ± 7.6 | 15.20 ± 1.2 |
| tHGA ** | 94.3 ± 3.4 | 23.61 ± 1.7 |
| NDGA *** | 100.0 ± 0.0 | 0.10 ± 0.0 |
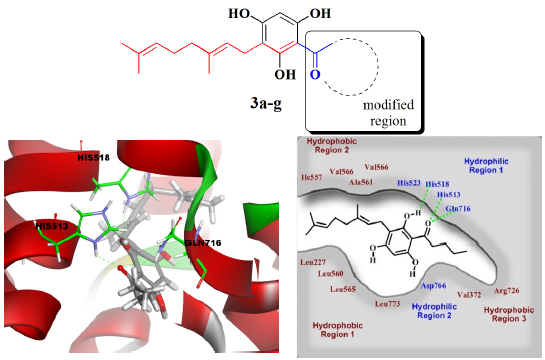
2.3. Molecular Docking


3. Experimental Section
3.1. Synthesis of 2,4,6-Trihydroxy-3-Geranylacetophenone (tHGA) Analogs
3.1.1. General Methods
3.1.2. Friedel-Craft Acylation
3.1.3. Friedel-Craft Alkylation
3.2. In Vitro Soybean 15-Lipoxygenase (LOX) Inhibition Assay

3.3. Molecular Docking
4. Conclusions
Supplementary Files
Supplementary File 1Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gardner, H.W. Recent investigations into the lipoxygenase pathway in plants. Biochim. Biophys. Acta 1991, 1084, 221–239. [Google Scholar] [CrossRef]
- Assadieskandar, A.; Amini, M.; Salehi, M.; Sadeghian, M.; Sakhteman, A.; Nadri, H.; Shafiee, A. Synthesis and SAR study of 4,5-diaryl-1H-imidazole-2(3H)-thione derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2012, 20, 7160–7166. [Google Scholar] [CrossRef]
- Larsen, J.S.; Acosta, E.P. Leukotriene-receptor antagonists and 5-lipoxygenase inhibitors in asthma. Ann. Pharmacother. 1993, 27, 898–903. [Google Scholar]
- Aparoy, P.; Reddy, R.N.; Guruprasad, L.; Reddy, M.R.; Reddanna, P. Homology modeling of 5-lipoxygenase and hints for better inhibitor design. J. Comput. Aid. Mol. Des. 2008, 22, 611–619. [Google Scholar] [CrossRef]
- McGimley, C.M; van der Donk, W.A. Enzymatic hydrogen atom abstraction from polyunsaturated fatty acids. Chem. Commun. 2003, 23, 2843–2846. [Google Scholar]
- Wisastra, R.; Ghizzoni, M.; Boltjes, A.; Haisma, H.J.; Dekker, F.J. Anacardic acid derived salicylates are inhibitors or activators of lipoxygenases. Bioorg. Med. Chem. 2012, 20, 5027–5032. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar]
- Schewe, T. 15-lipoxygenase-1: A prooxidant enzyme. Biol. Chem. 2002, 383, 365–374. [Google Scholar] [CrossRef]
- Kelavkar, U.P.; Cohen, C.; Kamitani, H.; Eling, T.E.; Badr, K.F. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: Correlation with Gleason staging. Carcinogenesis 2000, 21, 1777–1787. [Google Scholar] [CrossRef]
- Zhu, J.; Kilty, I.; Granger, H.; Gamble, E.; Qiu, Y.S.; Hattotuwa, K.; Elston, W.; Liu, W.L.; Olivia, A.; Pauwels, R.A.; et al. Gene expression and immunolocalization of 15-lipoxygenase isoenzymes in the airway mucosa of smokers with chronic bronchitis. Am. J. Resp. Cell Mol. Biol. 2002, 27, 666–677. [Google Scholar] [CrossRef]
- Sultana, C.; Shen, Y.; Rattan, V. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. Cell Phys. 1996, 167, 477–487. [Google Scholar] [CrossRef]
- Setty, B.N.; Werner, M.H.; Annun, Y.A.; Stuart, M.J. 15-hydroxyeicosatetraenoic acid-mediated potentiation of thrombin-induced platelet functions occurs via enhanced production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol-1,4,5-triphosphate. Blood 1992, 80, 2765–2773. [Google Scholar]
- Dahlén, S.E. Treatment of asthma with antileukotrienes: First line or last resort therapy? Eur. J. Pharmacol. 2006, 533, 40–56. [Google Scholar] [CrossRef]
- Suryati, S. Phytochemicals and Anti-Inflammatory Activity of Melicope ptelefolia Champ Ex Benth. Master’s Thesis, Universiti Putra Malaysia, Malaysia, 2005. [Google Scholar]
- Shaari, K.; Safri, S.; Abas, F.; Lajis, N.H.; Israf, D.A. A geranylacetophenone from the leaves of Melicope ptelefolia. Nat. Prod. Lett. 2006, 20, 415–419. [Google Scholar]
- Shaari, K.; Johnson, S.; Velan, S.; Seema, Z.; Faridah, A.; Daud, I.A.; Nordin, L. Leukotriene Inhibitor and Method for Producing the Same. PI2010000968. 31 December 2010. [Google Scholar]
- Shaari, K.; Suppaiah, V.; Wai, L.K.; Stanslas, J.; Tejo, B.A.; Israf, D.A.; Abas, F.; Safri, S.; Shuaib, H.; Zareen, S.; et al. Bioassay-guided identification of an anti-inflammatory prenylated acylphloroglucinol from Melicope ptelefolia and molecular insights into its interaction with 5-lipoxygenase. Bioorg. Med. Chem. 2011, 19, 6340–6347. [Google Scholar] [CrossRef]
- Ismail, N.; Jambari, N.N.; Zareen, S.; Akhtar, M.N.; Shaari, K.; Zamri-Saad, M.; Ling, T.C.; Sulaiman, M.R.; Lajis, N.H.; Israf, D.A. A geranyl acetophenone targeting cysteinyl leukotriene synthesis prevents allergic airway inflammation in ovalbumin-sensitized mice. Toxicol. Appl. Pharmacol. 2012, 259, 257–262. [Google Scholar] [CrossRef]
- Chung, K.F. Leukotriene receptor antagonists and biosynthesis inhibitors: Potential breakthrough in asthma therapy. Eur. Respir. J. 1995, 8, 1203–1213. [Google Scholar] [CrossRef]
- Crockett, S.L.; Wenzig, E.M.; Kunert, O.; Bauer, R. anti-inflammatory phloroglucinol derivatives from Hypericum empetrifolium. Phytochem. Lett. 2008, 1, 37–43. [Google Scholar] [CrossRef]
- Wecksler, A.T.; Garcia, N.K.; Holman, T.R. Substrate specificity effects of lipoxygenase products and inhibitors on soybean lipoxygenase-1. Bioorg. Med. Chem. 2009, 17, 6534–6539. [Google Scholar] [CrossRef]
- Hartl, A.; Reininger, W. Method of Acylation of Phloroglucinol. U.S. Patent 4,053,517, 11 October 1977. [Google Scholar]
- Kubo, I.; Ha, T.J.; Shimizu, K. Molecular design of soybean lipoxygenase inhibitors based on natural products. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Graham, L.P. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press: New York, NY, USA, 2009; pp. 212–222. [Google Scholar]
- Skrzypczak-Jankun, E.; Chorostowska-Wynimko, J.; Selman, S.H.; Jankun, J. Lipoxygenases—A challenging problem in enzyme inhibition and drug development. Curr. Enzyme Inhib. 2007, 3, 119–132. [Google Scholar] [CrossRef]
- Boyington, J.C.; Gaffney, B.J.; Amzel, L.M. The three-dimensional structure of an arachidonic acid 15-lipoxygenase. Science 1993, 260, 1482–1486. [Google Scholar]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Skrzypczak-Jankun, E.; Bross, R.A.; Carroll, R.T.; Dunham, W.R.; Funk, M.O. Three-dimensional structure of a purple lipoxygenase. J. Am. Chem. Soc. 2001, 123, 10814–10820. [Google Scholar] [CrossRef]
- Rullah, K.; Aluwi, M.F.F.; Yamin, B.M.; Bohari, M.N.A.; Wei, L.S.; Ahmad, S.; Abas, F.; Ismail, N.H.; Jantan, I.; Wai, L.K. Inhibition of prostaglandin E2 production by synthetic minor prenylated chalcones and flavonoids: Synthesis, biological activity, crystal structure, and in silico evaluation. Bioorg. Med. Chem. Lett. 2014. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ng, C.H.; Rullah, K.; Aluwi, M.F.F.M.; Abas, F.; Lam, K.W.; Ismail, I.S.; Narayanaswamy, R.; Jamaludin, F.; Shaari, K. Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor. Molecules 2014, 19, 11645-11659. https://doi.org/10.3390/molecules190811645
Ng CH, Rullah K, Aluwi MFFM, Abas F, Lam KW, Ismail IS, Narayanaswamy R, Jamaludin F, Shaari K. Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor. Molecules. 2014; 19(8):11645-11659. https://doi.org/10.3390/molecules190811645
Chicago/Turabian StyleNg, Chean Hui, Kamal Rullah, Mohd Fadhlizil Fasihi Mohd Aluwi, Faridah Abas, Kok Wai Lam, Intan Safinar Ismail, Radhakrishnan Narayanaswamy, Fadzureena Jamaludin, and Khozirah Shaari. 2014. "Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor" Molecules 19, no. 8: 11645-11659. https://doi.org/10.3390/molecules190811645
APA StyleNg, C. H., Rullah, K., Aluwi, M. F. F. M., Abas, F., Lam, K. W., Ismail, I. S., Narayanaswamy, R., Jamaludin, F., & Shaari, K. (2014). Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor. Molecules, 19(8), 11645-11659. https://doi.org/10.3390/molecules190811645