Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE)
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis
| Compound | RT (min) | RIexp | RIlit | % RA | Identification |
|---|---|---|---|---|---|
| δ-Elemene | 21.18 | 1344 | 1339 | 0.5 | RI, MS |
| α-Ylangene | 22.52 | 1379 | 1372 | 0.6 | RI, MS |
| δ-Copaene | 22.69 | 1383 | 1376 | 0.6 | RI, MS |
| β-Elemene | 23.37 | 1401 | 1393 | 3.2 | RI, MS |
| α-Gurjunene | 23.66 | 1408 | 1409 | 3.3 | RI, MS |
| β-Caryophyllene | 24.44 | 1429 | 1428 | 24.9 | RI, MS |
| trans-α-Bergamotene | 24.66 | 1435 | 1436 | 4.9 | RI, MS |
| α-Guaiene | 24.96 | 1443 | 1439 | 4.2 | RI, MS |
| α-Humulene | 25.34 | 1453 | 1455 | 2.6 | RI, MS |
| allo-Aromadendrene | 25.75 | 1464 | 1461 | 7.5 | RI, MS |
| γ-Gurjunene | 26.26 | 1478 | 1477 | 0.3 | RI, MS |
| 9-epi-Caryophyllene | 26.49 | 1484 | 1478 | 0.8 | RI, MS |
| Germacrene D | 26.56 | 1486 | 1480 | 0.3 | RI, MS |
| β-Selinene | 26.66 | 1488 | 1485 | 0.9 | RI, MS |
| Germacrene B | 26.99 | 1497 | 1499 | 5.1 | RI, MS |
| α-Muurolene | 27.08 | 1500 | 1499 | 2.6 | RI, MS |
| β-Bisabolene | 27.32 | 1506 | 1509 | 6.3 | RI, MS |
| δ-Amorphene | 27.46 | 1510 | 1512 | 4.8 | RI, MS |
| γ-Cadinene | 27.68 | 1516 | 1513 | 0.8 | RI, MS |
| δ-Cadinene | 27.91 | 1523 | 1524 | 15.3 | RI, MS |
| β-Sesquiphellandrene | 28.05 | 1527 | 1524 | 1.1 | RI, MS |
| α-Cadinene | 28.32 | 1534 | 1531 | 5.6 | RI, MS |
| Selina-3,7-(11)-diene | 28.78 | 1547 | 1545 | 0.1 | RI, MS |
| Elemol | 28.54 | 1540 | 1547 | 0.5 | RI, MS |
| Caryophyllenyl alcohol | 28.95 | 1552 | 1550 | 4.7 | RI, MS |
| Hydrocarbon sesquiterpenes | 94.8 | ||||
| Oxygenated sesquiterpenes | 5.2 |

2.2. In Vitro Effect of Copaiba Oil in Splenocyte Culture
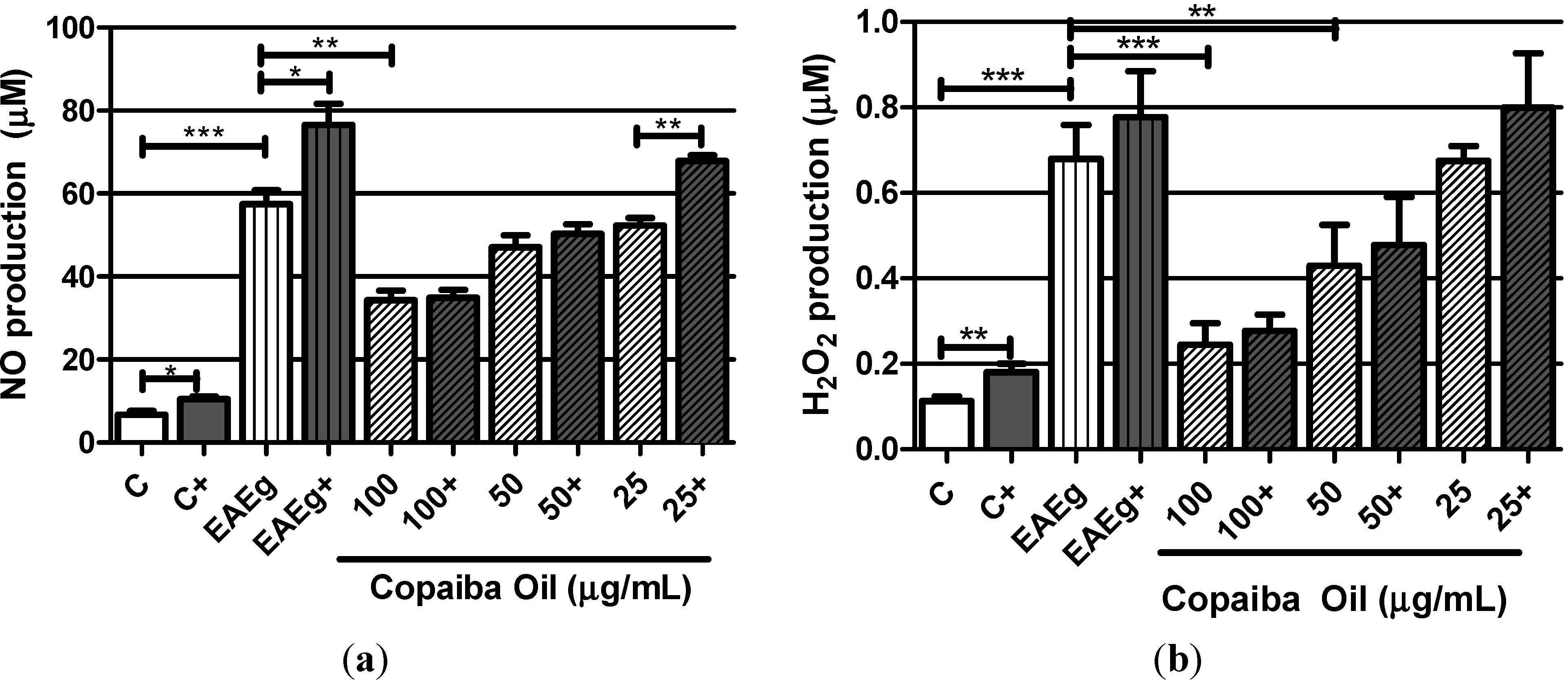
2.2.1. Levels of Oxygen Radicals (NO and H2O2)
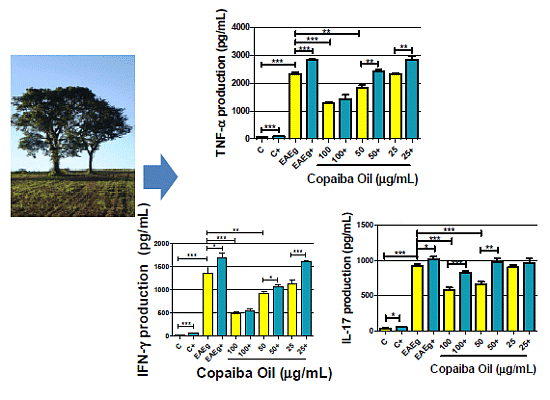
2.2.2. Cytokine Production

2.2.3. Discussion
3. Experimental
3.1. Copaiba Oil
3.2. CG Analysis
3.3. Animals
3.4. EAE Induction
3.5. Obtainment of Splenocytes, Culture and Treatments
3.6. H2O2 Measurement
3.7. Nitric Oxide (NO) Measurement
3.8. Determination of Cytokines Production
3.9. Statistics Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Ishikawa, L.L.W.; da Rosa, L.C.; Marques, C.; Ikoma, M.R.V.; Sartori, A. Persistent inflammation in the CNS during chronic EAE despite local absence of Il-17 production. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Gasperini, C.; Ruggieri, S.; Mancinelli, C.R.; Pozzilli, C. Advances in the treatment of relapsing-remitting multiple sclerosis—Critical appraisal of Fingolimod. Ther. Clin. Risk Manag. 2013, 9, 73–85. [Google Scholar] [PubMed]
- Corrêa, J.O.D.A.; Aarestrup, B.J.V.; Aarestrup, F.M. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2010, 226, 15–23. [Google Scholar]
- Alves, C.C.S.; Castro, S.B.R.; Costa, C.F.; Dias, A.T.; Alves, C.J.; Rodrigues, M.F.; Teixeira, H.C.; Almeida, M.V.; Ferreira, A.P. Anthraquinone derivative o,oꞌ-bis-(3ꞌ-iodopropyl)-1,4-dihidroxyanthraquinone modulates immune response and improves experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2012, 14, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Pöllinger, B. IL-17 producing T cells in mouse models of multiple sclerosis and rheumatoid arthritis. J. Mol. Med. 2012, 90, 613–624. [Google Scholar]
- Guimarães-Santos, A.; Santos, D.S.; Santos, I.R.; Lima, R.R.; Pereira, A.; de Moura, L.S.; Carvalho, R.N., Jr.; Lameira, O.; Gomes-Leal, W. Copaiba oil-resin treatment is neuroprotective and reduces neutrophil recruitment and microglia activation after motor cortex excitotoxic injury. Evid.-Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Da Silva Filho, A.A.; Bueno, P.C.P.; Gregório, L.E.; Silva, M.L.A.; Albuquerque, S.; Bastos, J.K. In vitro trypanocidal activity evaluation of crude extract and isolated compounds from Baccharis dracunculifolia D.C. (Asteraceae). J. Pharm. Pharmacol. 2004, 56, 1195–1199. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar]
- Leandro, L.M.; Vargas, F.S.; Barbosa, P.C.S.; Neves, J.K.O.; Silva, J.A.; Veiga-Junior, V.F. Chemistry and biological activities of terpenoids from Copaiba (Copaifera spp.) oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar]
- Veiga-Junior, V.F.; Zunino, L.; Calixto, J.B.; Patitucci, M.L.; Pinto, A.C. Phytochemical and antioedematogenic studies of commercial copaiba oils available in Brazil. Phytother. Res. 2001, 15, 476–480. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2005; p. 4. [Google Scholar]
- Deus, R.J.A.; Carvalho, A.S.C.; Banna, D.A.D.S.; Arruda, M.S.P.; Alves, C.N.; Santos, A.S. In vitro fungitoxic effect of the oil-resin and the essential oil of copaíba (Copaifera multijuga Hayne). Rev. Bras. Plantas Med. 2009, 11, 347–353. [Google Scholar]
- Barbosa, P.C.S.; Medeiros, R.S.; Sampaio, P.T.B.; Vieira, G.; Wiedemann, L.S.M.; Veiga-Júnior, V.F. Influence of abiotic factors on the chemical composition of copaíba oil (Copaifera multijuga Hayne): Soil composition, seasonality and diameter at breast height. J. Braz. Chem. Soc. 2012, 23, 1823–1833. [Google Scholar] [CrossRef]
- Trindade, F.T.T.; Stabeli, R.G.; Pereira, A.A.; Facundo, V.A.; Silva, A.A. Copaifera multijuga ethanolic extracts, oilresin and its derivatives display larvicidal activity against Anopheles darlingi and Aedes aegypti (Diptera: Culicidae). Rev. Bras. Farmacogn. 2013, 23, 464–470. [Google Scholar] [CrossRef]
- Chen, X.; Pi, R.; Zou, Y.; Liu, M.; Ma, X.; Jiang, Y.; Mao, X.; Hu, X. Attenuation of experimental autoimmune encephalomyelitis in C57 bl/6 mice by osthole, a natural coumarin. Eur. J. Pharmacol. 2010, 629, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; de Cezaro de Souza, P.R.; Bento, A.F.; Marcon, R.; Bicca, M.A.; Pianowski, L.F.; Calixto, J.B. Euphol prevents experimental autoimmune encephalomyelitis in mice: Evidence for the underlying mechanisms. Biochem. Pharmacol. 2012, 83, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Maffei Facino, R. GC-MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar]
- Barfod, L.; Kemp, K.; Hansen, M.; kharazmi, A. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int. Immunopharmacol. 2002, 2, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, J.; Funakoshi-Tago, M.; Tago, K.; Mashino, T.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-κB p65 phosphorylation at serine 276. Cell. Signal. 2009, 21, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tago, K.; Nishizawa, C.; Takahashi, K.; Mashino, T.; Iwata, S.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A is a potent inhibitor of TEL-Jak2-mediated transformation through the specific inhibition of Stat3 activation. Biochem. Pharmacol. 2008, 76, 1681–1693. [Google Scholar]
- Castro, S.B.R.; Junior, C.O.R.; Alves, C.C.S.; Dias, A.T.; Alves, L.L.; Mazzoccoli, L.; Mesquita, F.P.; Figueiredo, N.S.V.; Juliano, M.A.; Castañon, M.C.M.N.; et al. Immunomodulatory effects and improved prognosis of experimental autoimmune encephalomyelitis after O-tetradecanoyl-genistein treatment. Int. Immunopharmacol. 2012, 12, 465–470. [Google Scholar]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2010, 34, 678–692. [Google Scholar]
- Leiper, J.; Nandi, M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat. Rev. Drug Discov. 2011, 10, 277–291. [Google Scholar]
- Lee, E.; Chanamara, S.; Pleasure, D.; Soulika, A.M. IFN-gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J. Neuroinflamm. 2012, 9, 7. [Google Scholar] [CrossRef]
- Rodgers, J.M.; Miller, S.D. Cytokine control of inflammation and repair in the pathology of multiple sclerosis. Yale J. Biol. Med. 2012, 85, 447–468. [Google Scholar]
- Wang, X.; Ma, C.; Wu, J.; Zhu, J. Roles of T helper 17 cells and Interleukin-17 in neuroautoimmune diseases with emphasis on multiple sclerosis and guillainbarr e syndrome as well as their animal models. J. Neurosci. Res. 2013, 91, 871–881. [Google Scholar]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Antiinflammatory cannabinoids in diet—Towards a better understanding of cb2 receptor action? Commun. Integr. Biol. 2008, 1, 26–28. [Google Scholar]
- Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, 8th ed.; Hoboken, N.J., Ed.; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Pick, E.; Mizel, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods 1981, 46, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dias, D.S.; Fontes, L.B.A.; Crotti, A.E.M.; Aarestrup, B.J.V.; Aarestrup, F.M.; Da Silva Filho, A.A.; Corrêa, J.O.A. Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE). Molecules 2014, 19, 12814-12826. https://doi.org/10.3390/molecules190812814
Dias DS, Fontes LBA, Crotti AEM, Aarestrup BJV, Aarestrup FM, Da Silva Filho AA, Corrêa JOA. Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE). Molecules. 2014; 19(8):12814-12826. https://doi.org/10.3390/molecules190812814
Chicago/Turabian StyleDias, Débora S., Lívia B. A. Fontes, Antônio E. M. Crotti, Beatriz J. V. Aarestrup, Fernando M. Aarestrup, Ademar A. Da Silva Filho, and José O. A. Corrêa. 2014. "Copaiba Oil Suppresses Inflammatory Cytokines in Splenocytes of C57Bl/6 Mice Induced with Experimental Autoimmune Encephalomyelitis (EAE)" Molecules 19, no. 8: 12814-12826. https://doi.org/10.3390/molecules190812814