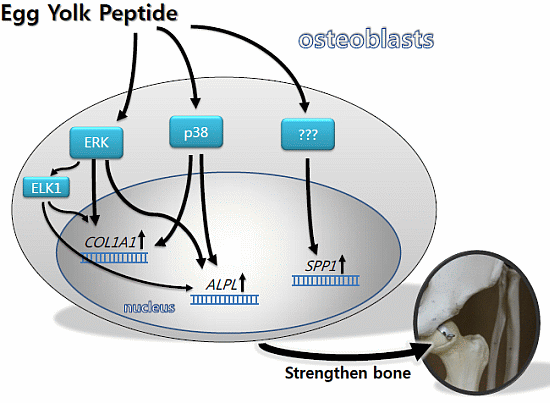
Effects of Egg Yolk-Derived Peptide on Osteogenic Gene Expression and MAPK Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. YPEP Characteristics
2.2. YPEP Enhances Osteogenesis
2.3. YPEP Activates MAPKs and ELK Pathway




2.4. YPEP Enhances Osteogenic Gene Expression



2.5. Discussion

3. Experimental Section
3.1. Preparation and Characteristics of Egg Yolk Water-Soluble Peptide (YPEP)
3.2. Cell Culture
3.3. Cell Proliferation and Alkaline Phosphatase Activity
3.4. Collagen Content
3.5. Calcium Deposition
3.6. Western Blot Analysis
3.7. Real-Time PCR
| Primer | Direction | Sequence |
|---|---|---|
| collagen, type I, alpha 1 (COL1A1) | Forward Reverse | 5'-GCG GCT CCC CAT TTT TAT ACC-3' 5'-GCT CTC CTC CCA TGT TAA ATA GCA-3' |
| alkaline phosphatase (ALPL) | Forward Reverse | 5'-AAA CCG AGA TAC AAG CAC TCC CAC-3' 5'-TCC GTC ACG TTG TTC CTG TTC AG-3' |
| osteocalcin (BGLAP) | Forward Reverse | 5'-CCA TGA GAG CCC TCA CAC TCC TC-3' 5'-GCT TGG ACA CAA AGG CTG CAC-3' |
| osteopontin (SPP1) | Forward Reverse | 5'-AGG CTG ATT CTG GAA GTT CTG AGG-3' 5'-GAC TTA CTT GGA AGG GYC TGT GGG-3' |
| housekeeping gene (GAPDH) | Forward Reverse | 5'-TCA TCA ATG GAA ATC CCA TCA CC-3' 5'-TGG ACT CCA CGA CGT ACT CAG C-3' |
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anton, M.; Nan, F.; Nys, Y. Bioactive egg components and their potential uses. World Poult. Sci. J. 2006, 62, 429–438. [Google Scholar] [CrossRef]
- Ji, M.; Leem, K.H.; Kim, M.; Kim, H.K. Egg yolk soluble protein stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biosci. Biotechnol. Biochem. 2007, 71, 1327–1329. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.; Leem, K.H. Protective effect of egg yolk peptide on bone metabolism. Menopause 2011, 18, 307–313. [Google Scholar] [CrossRef]
- Meisel, H. Biochemical properties of regulatory peptides derived from milk proteins. Biopolymers 1997, 43, 119–128. [Google Scholar] [CrossRef]
- Meisel, H. Biochemical properties of peptides encrypted in bovine milk proteins. Curr. Med. Chem. 2005, 12, 1905–1919. [Google Scholar] [CrossRef]
- Ngo, D.H.; Ryu, B.; Vo, T.S.; Himaya, S.W.; Wijesekara, I.; Kim, S.K. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116. [Google Scholar] [CrossRef]
- Gerdhem, P. Osteoporosis and fragility fractures. Best Pract. Res. Clin. Rheumatol. 2013, 27, 743–755. [Google Scholar] [CrossRef]
- Bone, H. Future directions in osteoporosis therapeutics. Endocrinol. Metab. Clin. North Am. 2012, 41, 655–661. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef]
- Lai, C.F.; Chaudhary, L.; Fausto, A.; Halstead, L.R.; Ory, D.S.; Avioli, L.V.; Cheng, S.L. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem. 2001, 276, 14443–14450. [Google Scholar]
- Suzuki, A.; Guicheux, J.; Palmer, G.; Miura, Y.; Oiso, Y.; Bonjour, J.P.; Caverzasio, J. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone 2002, 30, 91–98. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Chiba, N.; Bandow, K.; Kakimoto, K.; Masuda, A.; Ohnishi, T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J. Bone Miner. Res. 2009, 24, 398–410. [Google Scholar] [CrossRef]
- Treisman, R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996, 8, 205–215. [Google Scholar] [CrossRef]
- Zagar, Y.; Chaumaz, G.; Lieberherr, M. Signaling cross-talk from Gbeta4 subunit to Elk-1 in the rapid action of androgens. J. Biol. Chem. 2004, 279, 2403–2413. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Hipskind, R.A.; Bilbe, G. MAP kinase signaling and gene expression in osteoblasts. Front. Biosci. 1998, 3, d804–d816. [Google Scholar]
- Kim, H.K.; Department of Food & Biotechnology of Hanseo University, Seosan 356-706, Korea. Unpublished work. 2014.
- Nakamura, H. Morpholgy, function, and differentiation of bone cells. J. Hard Tissue Biol. 2007, 16, 15–22. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, X.; Wan, C.; Zhao, Q.; Zhang, L.; Zhou, Q.; Deng, L. Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: Differential signalling via Akt and ERK. Cell Biochem. Funct. 2012, 30, 297–302. [Google Scholar] [CrossRef]
- Aubin, J.E.; Liu, F.; Malaval, L.; Gupta, A.K. Osteoblast and chondroblast differentiation. Bone 1995, 17, 77S–83S. [Google Scholar] [CrossRef]
- Nakayama, K.; Tamura, Y.; Suzawa, M.; Harada, S.; Fukumoto, S.; Kato, M.; Miyazono, K.; Rodan, G.A.; Takeuchi, Y.; Fujita, T. Receptor tyrosine kinases inhibit bone morphogenetic protein-Smad responsive promoter activity and differentiation of murine MC3T3-E1 osteoblast-like cells. J. Bone Miner. Res. 2003, 18, 827–835. [Google Scholar] [CrossRef]
- Hu, Y.; Chan, E.; Wang, S.X.; Li, B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology 2003, 144, 2068–2074. [Google Scholar] [CrossRef]
- Kozawa, O.; Hatakeyama, D.; Uematsu, T. Divergent regulation by p44/p42 MAP kinase and p38 MAP kinase of bone morphogenetic protein-4-stimulated osteocalcin synthesis in osteoblasts. J. Cell. Biochem. 2002, 84, 583–589. [Google Scholar] [CrossRef]
- You, J.; Reilly, G.C.; Zhen, X.; Yellowley, C.E.; Chen, Q.; Donahue, H.J.; Jacobs, C.R. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 2001, 276, 13365–13371. [Google Scholar]
- Naor, Z.; Benard, O.; Seger, R. Activation of MAPK cascades by G-protein-coupled receptors: The case of gonadotropin-releasing hormone receptor. Trends Endocrinol. Metab. 2000, 11, 91–99. [Google Scholar] [CrossRef]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R., Jr.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef]
- Verrecchia, F.; Wagner, E.F.; Mauviel, A. Distinct involvement of the Jun-N-terminal kinase and NF-kappaB pathways in the repression of the human COL1A2 gene by TNF-alpha. EMBO Rep. 2002, 3, 1069–1074. [Google Scholar] [CrossRef]
- Kajiya, M.; Shiba, H.; Fujita, T.; Ouhara, K.; Takeda, K.; Mizuno, N.; Kawaguchi, H.; Kitagawa, M.; Takata, T.; Tsuji, K.; et al. Brain-derived neurotrophic factor stimulates bone/cementum-related protein gene expression in cementoblasts. J. Biol. Chem. 2008, 283, 16259–16267. [Google Scholar] [CrossRef]
- Danciu, T.E.; Adam, R.M.; Naruse, K.; Freeman, M.R.; Hauschka, P.V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003, 536, 19193–19197. [Google Scholar]
- Hipskind, R.A.; Rao, V.N.; Mueller, C.G.; Reddy, E.S.; Nordheim, A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 1991, 354, 531–534. [Google Scholar] [CrossRef]
- Sunters, A.; Thomas, D.P.; Yeudall, W.A.; Grigoriadis, A.E. Accelerated cell cycle progression in osteoblasts overexpressing the c-fos proto-oncogene: Induction of cyclin A and enhanced CDK2 activity. J. Biol. Chem. 2004, 279, 9882–9891. [Google Scholar] [CrossRef]
- Leem, K.; Kim, M.; Kim, H.; Kim, M.; Lee, Y.; Kim, H.K. Effects of egg yolk protein on the longitudinal bone growth of adolescent male rats. Biosci. Biotechnol. Biochem. 2004, 68, 2388–2390. [Google Scholar] [CrossRef]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef]
- Ryoo, H.M.; Lee, M.H.; Kim, Y.J. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 2006, 366, 51–57. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Valentine, L.S. Polarization microscopic studies of connective tissue stained with Picro-Sirius Red FBA. Beitr. Pathol. 1973, 150, 174–187. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the YPEP is available from the authors and Pharmafoods International, Japan.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, H.K.; Kim, M.-G.; Leem, K.-H. Effects of Egg Yolk-Derived Peptide on Osteogenic Gene Expression and MAPK Activation. Molecules 2014, 19, 12909-12924. https://doi.org/10.3390/molecules190912909
Kim HK, Kim M-G, Leem K-H. Effects of Egg Yolk-Derived Peptide on Osteogenic Gene Expression and MAPK Activation. Molecules. 2014; 19(9):12909-12924. https://doi.org/10.3390/molecules190912909
Chicago/Turabian StyleKim, Hye Kyung, Myung-Gyou Kim, and Kang-Hyun Leem. 2014. "Effects of Egg Yolk-Derived Peptide on Osteogenic Gene Expression and MAPK Activation" Molecules 19, no. 9: 12909-12924. https://doi.org/10.3390/molecules190912909