Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin
Abstract
:1. Introduction
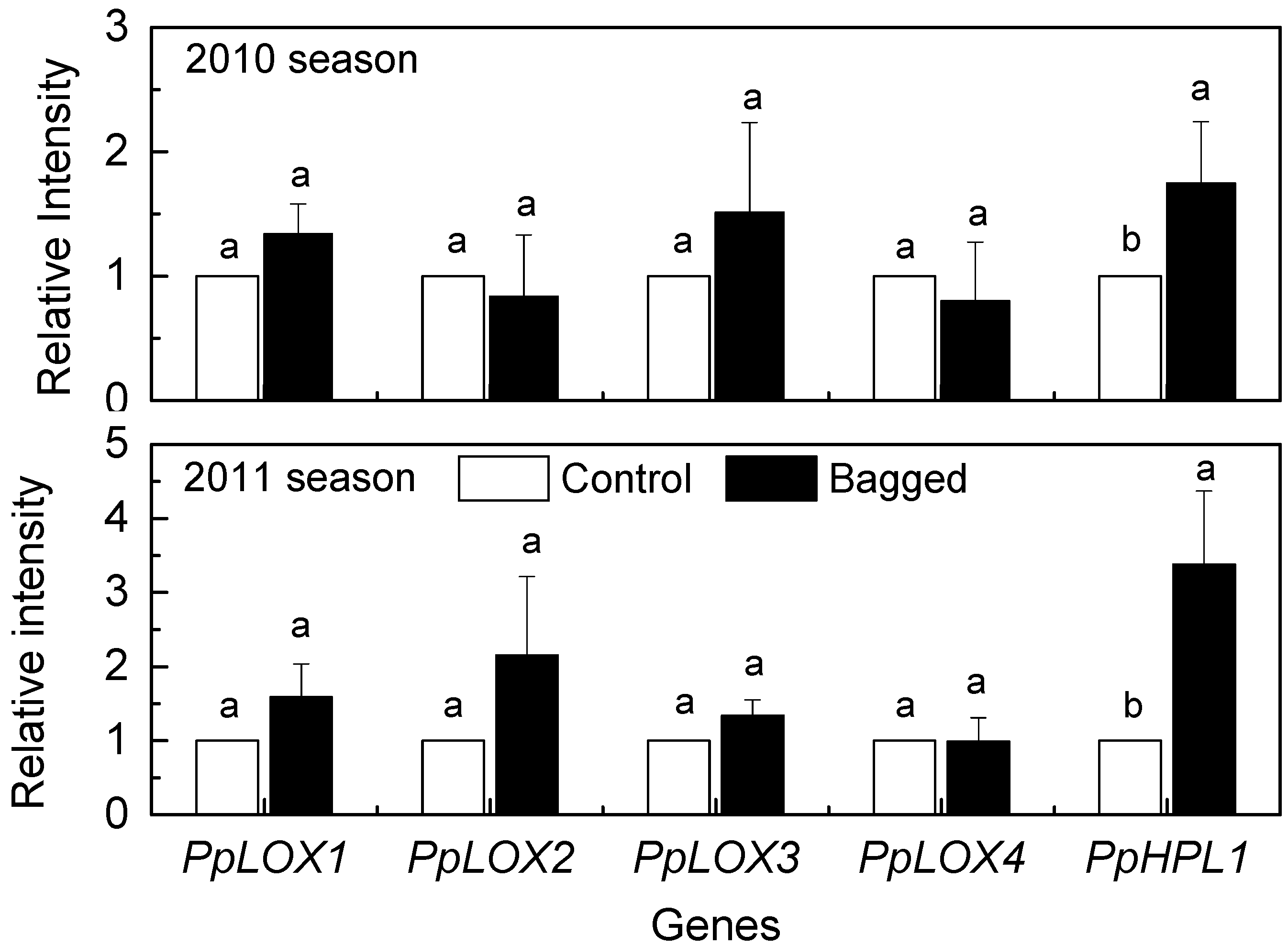
2. Results and Discussion
| 2010 Fruit Season | 2011 Fruit Season | |||
|---|---|---|---|---|
| Control | Bagged | Control | Bagged | |
| n-Hexanal | 64.03 ± 2.7 a | 95.58 ± 11.53 a | 26.98 ± 2.46 a | 39.49 ± 5.03 a |
| (E)-2-Hexenal | 39.98 ± 0.70 b | 63.63 ± 2.15 a | 12.10 ± 3.48 b | 45.09 ± 1.86 a |


| Volatiles | 2010 Fruit Season | 2011 Fruit Season | ||
|---|---|---|---|---|
| Control | Bagged | Control | Bagged | |
| Hexyl acetate | 2.28 ± 0.15 a | 1.97 ± 0.12 a | 0.13 ± 0.06 a | 0.06 ± 0.02 a |
| (Z)-3-Hexenyl acetate | 2.03 ± 0.26 a | 2.02 ± 0.33 a | 0.51 ± 0.25 a | 0.30 ± 0.03 a |
| (E)-2-Hexenyl acetate | 1.47 ± 0.22 a | 1.09 ± 0.15 a | 0.81 ± 0.21 a | 0.68 ± 0.09 a |
| γ-Octalactone | 3.13 ± 0.62 a | 2.42 ± 0.36 a | UD | UD |
| δ-Decalactone | UD | UD | 0.37 ± 0.02 a | 0.30 ± 0.04 a |
| γ-Decalactone | 0.41 ± 0.09 a | 0.32 ± 0.02 a | 1.09 ± 0.03 a | 0.87 ± 0.11 a |
| 2010 Fruit Season | 2011 Fruit Season | |||
|---|---|---|---|---|
| Control | Bagged | Control | Bagged | |
| Weight | 177.23 ± 6.51 a | 196.41 ± 3.10 a | 134.16 ± 4.34 a | 145.38 ± 4.98 a |
| L | 57.61 ± 1.06 b | 65.76 ± 0.68 a | 46.66 ± 0.78 b | 59.48 ± 1.22 a |
| Hab | 79.44 ± 2.22 b | 93.71 ± 0.75 a | 61.87 ± 0.39 b | 94.91 ± 0.68 a |
| C * | 20.52 ± 0.20 a | 21.77 ± 0.42 a | 20.90 ± 0.65 a | 22.26 ± 0.33 a |
| Chlorophyll a | 2.95 ± 0.49 a | 2.01 ± 0.35 a | NA | NA |
| Chlorophyll b | 1.77 ± 0.69 a | 1.02 ± 0.41 a | NA | NA |
| Firmness | 34.16 ± 1.11 a | 34.45 ± 2.69 a | NA | NA |
| TSS | 13.01 ± 0.31 a | 12.53 ± 0.45 a | NA | NA |
| Juiciness | 70.02 ± 1.64 a | 68.15 ± 2.44 a | NA | NA |
3. Experimental
3.1. Plant Materials and Sampling
3.2. Fruit Quality Evaluation
3.3. Fruit Volatile Analysis
3.4. Enzymes Activity Analysis
3.5. RNA Extraction and Gene Expression Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Appendix

Conflicts of Interest
References
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar]
- Horvat, R.J.; Chapman, G.W.; Robertson, J.A.; Meredith, F.I.; Scorza, R.; Callahan, A.M.; Morgens, P. Comparison of the volatile compounds from several commercial peach cultivars. J. Agric. Food Chem. 1990, 38, 234–237. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Montero-Prado, P.; Bentayeb, K.; Nerin, C. Pattern recognition of peach cultivars (Prunus persica L.) from their volatile components. Food Chem. 2013, 138, 724–731. [Google Scholar]
- Sánchez, G.; Venegas-Caleron, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genomics 2013, 14, 343. [Google Scholar]
- Xi, W.P.; Zhang, B.; Shen, J.Y.; Sun, C.D.; Xu, C.J.; Chen, K.S. Intermittent warming alleviated the loss of peach fruit aroma-related esters by regulation of AAT during cold storage. Postharvest Biol. Technol. 2012, 74, 42–48. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; López, M.L.; Crisosto, C.H.; Echeverria, G. Volatile compound emissions and sensory attributes of ‘Big Top’ nectarine and ‘Early Rich’ peach fruit in response to a pre-storage treatment before cold storage and subsequent shelf-life. Postharvest Biol. Technol. 2013, 76, 152–162. [Google Scholar]
- Ortiz, A.; Graell, J.; López, M.L.; Echeverria, G.; Lara, I. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 2010, 123, 698–704. [Google Scholar] [CrossRef]
- Yang, D.S.; Balandran-Quintana, R.R.; Ruiz, C.F.; Toledo, R.T.; Kays, S.J. Effect of hyperbaric, controlled atmosphere, and UV treatments on peach volatiles. Postharvest Biol. Technol. 2009, 52, 334–341. [Google Scholar]
- Jia, H.J.; Araki, A.; Okamoto, G. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica Batsch). Postharvest Biol. Technol. 2005, 35, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, C.X.; Liu, C.Y.; Xu, M.; Li, S.H.; Yang, L.; Wang, Y.N. Effects of bagging on volatiles and polyphenols in “Wanmi” peaches during endocarp hardening and final fruit rapid growth stages. J. Food Sci. 2010, 75, S455–S460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Che, F.; Wang, L.X.; Meng, R.; Zhang, X.J.; Zhao, Z.Y. Fruit coloration and anthocyanin biosynthesis after bag removal in non-red and red apples (Malus × domestica Borkh.). Molecules 2013, 18, 1549–1563. [Google Scholar]
- Huang, C.H.; Yu, B.; Teng, Y.W.; Su, J.; Shu, Q.; Cheng, Z.Q.; Zeng, L.Q. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci. Hortic. 2009, 121, 149–158. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxidelyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; Tsesmetzis, N.; di Sansebastiano, G.P.; Hughes, R.K.; Casey, R.; Santino, A. Subcellular localisation of Medicago truncatula 9/13-hydroperoxide lyase reveals a new localisation pattern and activation mechanism for CYP74C enzymes. BMC Plant Biol. 2007, 7, 58. [Google Scholar]
- Defilippi, B.G.; Manriquez, D.; Luengwilai, K.; Gonzalez-Aguero, M. Aroma volatiles: Biosynthesis and mechanisms of modulation during fruit ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xi, W.P.; Wei, W.W.; Shen, J.Y.; Ferguson, I.; Chen, K.S. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Bureau, S.M.; Razungles, A.J.; Baumes, R.L. The aroma of Muscat of Frontignan grapes: Effect of the light environment of vine or bunch on volatiles and glycoconjugates. J. Sci. Food Agric. 2000, 80, 2012–2020. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Omiya, A. Changes in the composition and content of volatile constituents in peach fruits in relation to maturity at harvest and artificial ripening. J. Jpn. Soc. Hortic. 1991, 60, 209–216. [Google Scholar] [CrossRef]
- Bertoli, A.; Lucchesini, M.; Mensuali-Sodi, A.; Leonardi, M.; Doveri, S.; Magnabosco, A.; Pistelli, L. Aroma characterization and UV elicitation of purple basil from different plant tissue cultures. Food Chem. 2013, 141, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Falara, V.; Amarasinghe, R.; Poldy, J.; Pichersky, E.; Barrow, R.A.; Peakall, R. The production of a key floral volatile is dependent on UV light in a sexually deceptive orchid. Ann. Bot. 2013, 111, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, T.A.; Schwieterman, M.L.; Gilbert, J.L.; Jawoski, E.A.; Langer, K.M.; Jones, C.R.; Rushing, G.V.; Hunter, T.M.; Olmstead, J.; Clark, D.G.; et al. Light modulation of volatile organic compounds from petunia flowers and selected fruits. Postharvest Biol. Technol. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Farcuh, M.; Meneses, C. Monitoring the sensorial quality and aroma through an electronic nose in peaches during cold storage. J. Sci. Food Agric. 2008, 88, 2073–2078. [Google Scholar] [CrossRef]
- Lewandowsk, M.; Jarvis, P.G. Changes in chlorophyll and carotenoid content, specific leaf area and dry weight fraction in sitka spruce, in response to shading and season. New Phytol. 1977, 79, 247–256. [Google Scholar] [CrossRef]
- Echeverria, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of “Fuji” apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Fukushige, H.; Hildebrand, D.F. Watermelon (Citrullus lanatus) hydroperoxide lyase greatly increases C-6 aldehyde formation in transgenic leaves. J. Agric. Food Chem. 2005, 53, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, K.S.; Bowen, J.; Allan, A.; Espley, R.; Karunairetnam, S.; Ferguson, I. Differential expression within the LOX gene family in ripening kiwifruit. J. Exp. Bot. 2006, 57, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the peach fruit are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shen, J.-Y.; Wu, L.; Liu, H.-R.; Zhang, B.; Yin, X.-R.; Ge, Y.-Q.; Chen, K.-S. Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin. Molecules 2014, 19, 13461-13472. https://doi.org/10.3390/molecules190913461
Shen J-Y, Wu L, Liu H-R, Zhang B, Yin X-R, Ge Y-Q, Chen K-S. Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin. Molecules. 2014; 19(9):13461-13472. https://doi.org/10.3390/molecules190913461
Chicago/Turabian StyleShen, Ji-Yuan, Lei Wu, Hong-Ru Liu, Bo Zhang, Xue-Ren Yin, Yi-Qiang Ge, and Kun-Song Chen. 2014. "Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin" Molecules 19, no. 9: 13461-13472. https://doi.org/10.3390/molecules190913461
APA StyleShen, J.-Y., Wu, L., Liu, H.-R., Zhang, B., Yin, X.-R., Ge, Y.-Q., & Chen, K.-S. (2014). Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin. Molecules, 19(9), 13461-13472. https://doi.org/10.3390/molecules190913461






