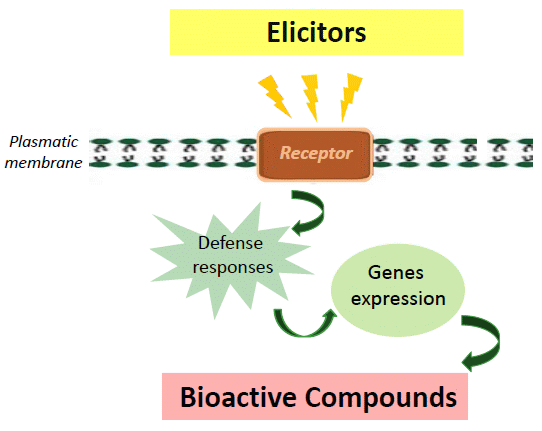
Elicitation: A Tool for Enriching the Bioactive Composition of Foods
Abstract
:1. Introduction: Secondary Metabolites in Plants, Foods and Human Health
2. Elicitors
2.1. Concept and Classification
| Biotic Elicitors | |
| Lipopolysaccharides [27] | |
| Polysaccharides: Pectin and cellulose (cell walls) [28]; chitosan [21,28], chitin and glucans (microorganisms) [28], alginate, arabic gum [29], guar gum, LBG [27], yeast extract [27]. | |
| Oligosaccharides: Galacturonides, guluronate, mannan, mannuronate [27,30]. | |
| Proteins: Cellulase [31], cryptogein [32], glycoproteins [27], oligandrin [27], pectolyase, fish protein hydrolysates [33], lactoferrin [33]. | |
| Complex composition: Fungal spores, mycelia cell wall, microbial cell wall [27]. | |
| Pathogen toxin: Coronatine [34]. | |
| Oregano extract [33]. | |
| Abiotic Elicitors | |
| Chemical | Physical [35] |
| Acetic acid [21] | Altered gas composition |
| Benzothiadiazole [36] | Chilling |
| Silicon [36] | CO2 |
| Bioregulator prohexadione | Drought |
| Ethanol [37] | Extreme temperature shock |
| Ethene [37] | High pressure |
| Inorganic salts: mercuric chloride (HgCl2), copper sulfate (CuSO4), calcium chloride (CaCl2), and vanadyl sulfate (VSO4) [28] | High or low osmolarity UV irradiation Saline stress |
| Metal ions: Co2+, Fe2+, Al3+, Ag2+, Ag+, Mn2+, Zn2+, Cu2+, Pb2+ and Cd2+ [28,38] | Wounding Ozone |
| Plant Hormones | |
| Jasmonic acid, methyl jasmonate [39], methyl salicylate, salicylic acid, ethylene [21,40], cytokinin, gibberellin GA3 [37]. | |
2.2. Mode of Action of Elicitors
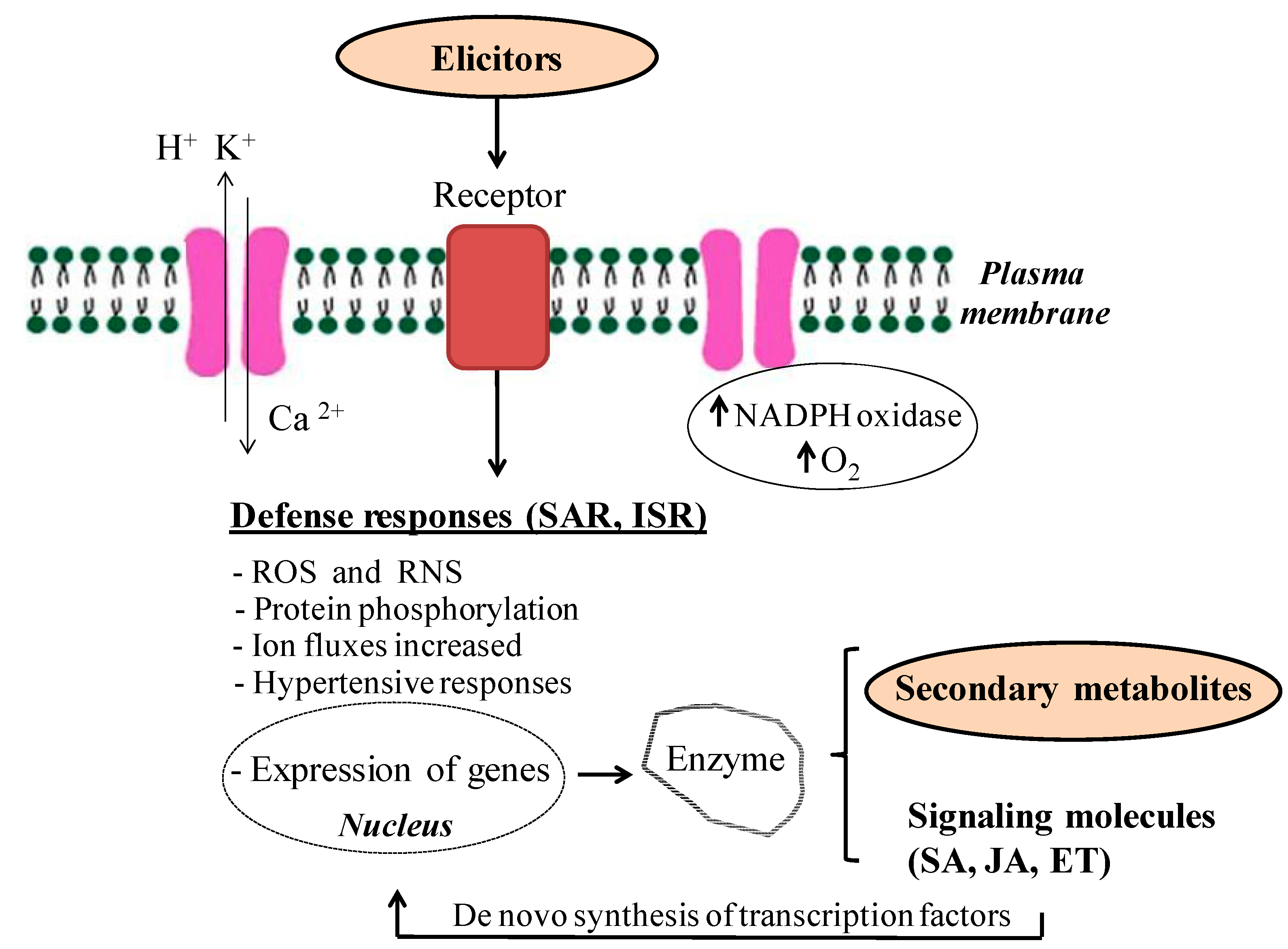
2.3. Preharvest Elicitation: Priming Seeds and Edible Plants

2.4. Postharvest Elicitors Applications
3. Elicitation Effects on Primary Metabolism
4. Elicitors Affecting the Content of Bioactive Compounds
4.1. Phenolic Compounds
| Plant Food | Elicitor Treatment | Application | Target Compounds Class and Increase | Reference |
|---|---|---|---|---|
| “Fuji” apples | Ethephon (2-chloroethyl phosphonic acid) (100 mg/L) | Sprayed for 4 weeks before commercial harvest | Anthocyanins (8-fold), and flavonols (2-fold) during fruit maturation | [90] |
| Grape berry fruits | Ethanol (5 g/100 mL) | Sprayed for 8–9 weeks after anthesis | Anthocyanins (3-fold) | [91] |
| Butter Lettuce | JA 1 µM | Sprayed after 21 days of germination | Total phenolics (280%) Flavonoids (133%) Phenolic acids (360%) | [92] |
| Lettuce cv. “Lollo Rosso” | UV-full range (UV-A and UV-B) | Radiation during cultivation | Flavonoids (130%) and phenolic acids (200%) | [93] |
| Purple-flesh potatoes | Wounding (vegetable slicer) | After harvest | Total phenolics (60%) | [94] |
| Strawberry fruits | CO2 (ambient + 600 µmol) | 28 months | Anthocyanin and flavonols (30%–50%) | [95] |
| Sweet basil | MeJA 0.5 mM | Sprayed when the plants had five or six leaves | Rosmarinic acid (50%) and caffeic acid (38%) | [96] |
| Greek oregano | Chitosan oligosaccharides (50 and 200 mg/L) | Sprayed for 2 weeks prior to the anticipated flowering time | Phenolic acids and flavonoids (30%) | [97] |
| Pea sprouts | Folin acid (50 µM) and vitamin C (500 µM) solutions | Soaking seeds for 12–48 h | Total phenolic compounds (20%) | [98] |
| Pea sprouts | Folin acid (50 µM) and vitamin C (500 µM) solutions | Soaking seeds for 12–48 h | Total phenolic compounds (20%) | [98] |
| Olive trees organs | Nutrient solution “Brotomax” (0.3 g/100 mL) (urea nitrogen, copper, manganese and zinc) | Sprayed for 120 days after anthesis | Tyrosol, catechin, and oleuropein (20%) | [99] |
| Radish sprouts | NaCl (100 mM) | In 0.5% agar media for 3, 5 and 7 days after sowing seeds | Total phenolics (30% and 50% in 5 and 7-days-old sprouts, respectively) | [100] |
| Radish, chinese kale and pak choi 3-day-old sprouts | Glucose (5 g/100 mL) | Hydroponic system for 3 days after sowing seeds | Total phenolics (20%) | [53] |
| Broccoli 7-day-old sprouts | Sucrose, fructose and glucose (146 mM) | In 0.5% agar media for 5 days after sowing seeds | Total anthocyanins (10%) | [55] |
| Broccoli 7-day-old sprouts | Sucrose and mannitol (176 mM) | Hydroponic system for 5 days after sowing seeds | Total anthocyanins (40%) and phenolics (50%) | [101] |
4.2. Glucosinolates
| Plant Food | Elicitor Treatment | Application | Target Compounds Class and Fold Increase | Reference |
|---|---|---|---|---|
| Brassica 7-day-old sprouts cotyledons and leaves | JA spray (5 nmol) | Topically | 3-indolylmethyl GLS (6-fold) in B. napus; 4-hydroxy-3-indolylmethyl GLS (9-fold) in B. rapa; both indole GLS (2-fold) in B. juncea | [56] |
| Turnip root exudates | MeJa (130 μM) | Added in the hydroponic system for 10 days | Indole GLS (4-fold) | [113] |
| Broccoli sprouts | Sucrose (146 mM) | In 0.5% agar media for 5 days after sowing seeds | Total GLS (2-fold) | [55] |
| Broccoli 7-day-old sprouts |
| Daily exogenous spraying during 3, 5 and 7 days |
| [25] |
| Radish, chinese kale and pak choi 3-day-old sprouts | Glucose (5 g/100 mL) | Hydroponic system for 3 days after sowing seeds | Gluconapin (150% and 60% in Chinese kale and pak choi, respectively) Glucobrassicanapin (110-fold in pak choi) | [53] |
| Sauerkraut (B. oleracea L. var. capitata) | 0.5% NaCl and 0.3 mg of sodium selenite/kg | Added to fresh cabbage before fermentation | Indole GLS hydrolysis products (indole-3- carbinol and indole-3- acetonitrile in 70% and 10%, respectively) | [114] |
| Radish sprouts | NaCl (100 mM) | In 0.5% agar media for 3, 5 and 7 days after sowing seeds | Total GLS (50% and 120% in 5 and 7-days-old sprouts, respectively) | [100] |
| Brassica 8-day-old sprotuts | MeJA (25 µM) JA (150 µM) Sucrose (146 mM) | Sprayed for 5 days before harvest | Total GLS Broccoli: >50% Turnip: >20% Rutabaga: >100% | [52] |
| Raphanus 8-day-old sprotuts | MeJA (25 µM) SA (100 µM) Glucose (277 mM) | Sprayed for 5 days before harvest | Total GLS: > 20% | [52] |
| Broccoli 7-day-old sprouts | Sucrose and mannitol (176 mM) | Hydroponic system for 5 days after sowing seeds | Total GLS: > 50% | [101] |
| Broccoli florets | Ethanol evaporated (500 μL/L) | 6 h after harvested | Total GLS: > 50% | [115] |
| Broccoli florets | MeJA spray (250 µM) | Aerial portions twice per week from flowering to head formation | Indolyl GLS: > 30% | [91,116] |
4.3. Carotenoids and Betalains
4.4. Nutrients with Biological Activity
5. Future Trends
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Guo, Y.L.; Zhang, P.Y.; Guo, M.R.; Chen, K.S. Secondary metabolites and plant defence against pathogenic disease. Plant Physiol. 2012, 48, 429–434. [Google Scholar]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Li, J.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to uv-b irradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; ASPP: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Pasini, F.; Verardo, V.; Cerretani, L.; Caboni, M.F.; D’Antuono, L.F. Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric. 2011, 91, 2858–2864. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Bjorkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Regulation (ec) no 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods. Official Journal of the European Union 2006. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:EN:PDF (accessed on 28 August 2014).
- Gundgaard, J.; Nielsen, J.N.; Olsen, J.; Sorensen, J. Increased intake of fruit and vegetables: Estimation of impact in terms of life expectancy and healthcare costs. Public Health Nutr. 2003, 6, 25–30. [Google Scholar]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Bellostas, N.; Kachlicki, P.; Sorensen, J.C.; Sorensen, H. Glucosinolate profiling of seeds and sprouts of B. oleracea varieties used for food. Sci. Hortic. 2007, 114, 234–242. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food ans significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products-a promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef]
- Pérez- Balibrea, S. Saline stress effect on the biochemistry of edible sprouts of broccoli (Brassica oleracea var italica). J. Clin. Biochem. Nutr. 2008, 43, 1–5. [Google Scholar]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.; Fanciullino, A.L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef]
- Poulev, A.; O’Neal, J.M.; Logendra, S.; Pouleva, R.B.; Timeva, V.; Garvey, A.S.; Gleba, D.; Jenkins, I.S.; Halpern, B.T.; Kneer, R.; et al. Elicitation, a new window into plant chemodiversity and phytochemical drug discovery. J. Med. Chem. 2003, 46, 2542–2547. [Google Scholar] [CrossRef]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures Food Biotechnology; Stahl, U., Donalies, U., Nevoigt, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 187–228. [Google Scholar]
- Keen, N.T. Specific elicitors of plant phytoalexin production: Determinants of race specificity in pathogens? Science 1975, 187, 74–75. [Google Scholar]
- Doughty, K.J.; Kiddle, G.A.; Pye, B.J.; Wallsgrove, R.M.; Pickett, J.A. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry 1995, 38, 347–350. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Caretto, S.; Nisi, R.; Paradiso, A.; de Gara, L. Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [Google Scholar] [CrossRef]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Mewis, I.; Knorr, D.; Smetanska, I. Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of vitis vinifera. Plant Cell Tiss. Org. 2012, 108, 401–409. [Google Scholar]
- Kobayashi, A.; Tai, A.; Kanzaki, H.; Kawazu, K. Elicitor-active oligosaccharides from algal laminaran stimulate the production of antifungal compounds in alfalfa. Z. Naturforsch 1993, 48c, 575–579. [Google Scholar]
- Ma, C. Cellulase elicitor induced accumulation of capsidiol in Capsicum annumm L. Suspension cultures. Biotechnol. Lett. 2008, 30, 961–965. [Google Scholar] [CrossRef]
- Mikes, V.; Milat, M.L.; Ponchet, M.; Ricci, P.; Blein, J.P. The fungal elicitor cryptogein is a sterol carrier protein. FEBS Lett. 1997, 416, 190–192. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar]
- Onrubia, M.; Moyano, E.; Bonfill, M.; Cusidó, R.M.; Goossens, A.; Palazón, J. Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J. Plant Physiol. 2013, 170, 211–219. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Dinh, S.Q.; Joyce, D.C.; Irving, D.E.; Wearing, A.H. Effects of multiple applications of chemical elicitors on botrytis cinerea infecting geraldton waxflower. Australas. Plant Pathol. 2008, 37, 87–94. [Google Scholar] [CrossRef]
- Treutter, D. Managing phenol contents in crop plants by phytochemical farming and breeding-visions and constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef]
- Suvarnalatha, G.; Rajendran, L.; Ravishankar, G.A.; Venkataraman, L.V. Elicitation of anthocyanin production in cell cultures of carrot (Daucus carota L.) by using elicitors and abiotic stress. Biotechnol. Lett. 1994, 16, 1275–1280. [Google Scholar]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar]
- Tomás-Barberán, F.A.; Loaiza-Velarde, J.; Bonfanti, A.; Saltveit, M.E. Early wound- and ethylene-induced changes in phenylpropanoid metabolism in harvested lettuce. J. Am. Soc. Hortic. Sci. 1997, 122, 399–404. [Google Scholar]
- Wang, S.Y.; Zheng, W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int. J. Food Sci. Technol. 2005, 40, 187–195. [Google Scholar] [CrossRef]
- Kondo, S.; Tomiyama, A. Changes of endogenous jasmonic acid and methyl jasmonate in apples and sweet cherries during fruit development. J. Am. Soc. Hort. Sci. 2000, 125, 282–287. [Google Scholar]
- Oh, M.-M.; Rajashekar, C.B. Antioxidant content of edible sprouts: Effects of environmental shocks. J. Sci. Food Agric. 2009, 89, 2221–2227. [Google Scholar] [CrossRef]
- Ebel, J.; Cosio, E.G. Elicitors of plant defense responses. Int. Rev. Cytol. 1994, 148, 1–36. [Google Scholar] [CrossRef]
- García-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe. Interact. 2006, 19, 711–724. [Google Scholar]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef]
- Ebel, J.; Mithöfer, A. Early events in the elicitation of plant defence. Planta 1998, 206, 335–348. [Google Scholar] [CrossRef]
- Ferrari, S. Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutics. Adv. Exp. Med. Biol. 2010, 698, 152–166. [Google Scholar] [CrossRef]
- Smetanska, I. Impact of elicitors on glucosinolate production in plants and exudates of turnip (Brassica rapa). Ph.D. Thesis, University of Berlin, Berlin, Germany, 2005. [Google Scholar]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Cho, M.H.; No, H.K.; Prinyawiwatkul, W. Chitosan treatments affect growth and selected quality of sunflower sprouts. J. Food Sci. 2008, 73, S70–77. [Google Scholar]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef]
- Wei, J.; Miao, H.; Wang, Q. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in brassica sprouts. Sci. Hortic.. 2011, 129, 535–540. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef]
- Bodnaryk, R.P.; Rymerson, R.T. Effect of wounding and jasmonates on the physico-chemical properties and flea beetle defence responses of canola seedlings, Brassica napus L. Can. J. Plant Sci. 1994, 74, 899–907. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. Italica) as conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, C673–C677. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar]
- Vallejo, F.; García-Viguera, C.; Tomás-Barberán, F.A. Changes in broccoli (Brassica oleracea L. var. italica) health-promoting compounds with inflorescence development. J. Agric. Food Chem. 2003, 51, 3776–3782. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Son, S.Y.; Rhee, H.S.; Yoon, S.-Y.H.; Lee-Parsons, C.W.T.; Park, J.M. Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J. Biotechnol. 2008, 135, 117–122. [Google Scholar] [CrossRef]
- Zhang, W.; Curtin, C.; Kikuchi, M.; Franco, C. Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci. 2002, 162, 459–468. [Google Scholar]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Milella, R.A.; Perniola, R.; Antonacci, D. Role of the physical elicitors in enhancing postharvest antioxidant capacity of table grape cv redglobe (Vitis vinifera L.). J. Food Res. 2014, 3. [Google Scholar] [CrossRef]
- Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Bilger, W.; Berge, A.; Haffner, K.; Solhaug, K.A. Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica borkh., cv. aroma): Effect of postharvest UV-B irradiation. Postharvest Biol. Technol. 2007, 45, 1–10. [Google Scholar]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. Food Qual. 2003, 77, 128–146. [Google Scholar]
- De la Peña Moreno, F.; Monagas, M.; Blanch, G.P.; Bartolomé, B.; Ruiz del Castillo, M.L. Enhancement of anthocyanins and selected aroma compounds in strawberry fruits through methyl jasmonate vapor treatment. Eur. Food Res. Technol. 2010, 230, 989–999. [Google Scholar] [CrossRef]
- Figueroa Pérez, M.G.; Rocha-Guzmán, N.E.; Mercado-Silva, E.; Loarca-Piña, G.; Reynoso-Camacho, R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014, 156, 273–278. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv groppello gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1564. [Google Scholar] [CrossRef]
- Shichijo, C.; Hamada, T.; Hiraoka, M.; Johnson, C.B.; Hashimoto, T. Enhancement of red-light-induced anthocyanin synthesis in sorghum first internodes by moderate low temperature given in the pre-irradiation culture period. Planta 1993, 191, 238–245. [Google Scholar]
- Heredia, J.B.; Cisneros-Zevallos, L. The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem. 2009, 115, 1500–1508. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Osmotic stress tolerance physiological and molecular considerations. Biotechnol. Plant. Acta Hort. (ISHS) 2001, 560, 285–292. [Google Scholar]
- Gómez, S.; Ferrieri, R.A.; Schueller, M.; Orians, C.M. Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol. 2010, 188, 835–844. [Google Scholar] [CrossRef]
- Hong, E.; Kang, H. Effect of smoke and aspirin stimuli on the germination and growth of alfalfa and broccoli. Electron. J. Environ. Agric. Food Chem. 2011, 10, 1918–1926. [Google Scholar]
- No, H.K.; Lee, K.S.; Kim, I.D.; Park, M.J.; Kim, S.D.; Meyers, S.P. Chitosan treatment affects yield, ascorbic acid content, and hardness of soybean sprouts. J. Food Sci. 2003, 68, 680–685. [Google Scholar] [CrossRef]
- Sato, F.; Hashimoto, T.; Hachiya, A.; Tamura, K.-I.; Choi, K.-B.; Morishige, T.; Fujimoto, H.; Yamada, Y. Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 367–372. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 2014. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ju, H.K.; Kim, Y.J.; Lim, T.G.; Uddin, M.R.; Kim, Y.B.; Baek, J.H.; Kwon, S.W.; Lee, K.W.; Seo, H.S.; et al. Enhancement of anti-inflammatory activity of aloe vera adventitious root extracts through the alteration of primary and secondary metabolites via salicylic acid elicitation. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Itoh, T.; Oyama, M.; Takimoto, N.; Kato, C.; Nozawa, Y.; Akao, Y.; Iinuma, M. Inhibitory effects of sesquiterpene lactones isolated from Eupatorium chinense L. on IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Bioorg. Med. Chem. 2009, 17, 3189–3197. [Google Scholar] [CrossRef]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the nurses’ health study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar]
- Sturgeon, S.R.; Ronnenberg, A.G. Pomegranate and breast cancer: Possible mechanisms of prevention. Nutr. Rev. 2010, 68, 122–128. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; López-Pérez, L.; Hernández, M.; López-Berenguer, C.; Fernández-García, N.; Carvajal, M. Agricultural practices for enhanced human health. Phytochem. Rev. 2008, 7, 251–260. [Google Scholar]
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. Crit. Rev. Plant Sci. 2006, 25, 267–278. [Google Scholar] [CrossRef]
- Li, Z.; Gemma, H.; Iwahori, S. Stimulation of ‘fuji’ apple skin color by ethephon and phosphorus–calcium mixed compounds in relation to flavonoid synthesis. Sci. Hortic. 2002, 94, 193–199. [Google Scholar] [CrossRef]
- El Kereamy, A.; Chervin, C.; Souquet, J.-M.; Moutounet, M.; Monje, M.-C.; Nepveu, F.; Mondies, H.; Ford, C.M.; van Heeswijck, R.; Roustan, J.-P. Ethanol triggers grape gene expression leading to anthocyanin accumulation during berry ripening. Plant Sci. 2002, 163, 449–454. [Google Scholar] [CrossRef]
- Złotek, U.; Świeca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bunce, J.A.; Maas, J.L. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef]
- Yin, H.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2011, 60, 136–143. [Google Scholar]
- Burguieres, E.; McCue, P.; Kwon, Y.-I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Del Rı́o, J.A.; Báidez, A.G.; Botı́a, J.M.; Ortuño, A. Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against phytophthora sp. Food Chem. 2003, 83, 75–78. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219–S219. [Google Scholar]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Kristal, A.R.; Lampe, J.W. Brassica vegetables and prostate cancer risk: A review of the epidemiological evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome p450 cyp79b2 from arabidopsix catalyzed the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar]
- Awang, N.A.; Islam, M.R.; Ismail, M.R.; Zulkarami, B.; Omar, D. Effectiveness of different elicitors in inducing resistance in chilli (Capsicum annuum L.) against pathogen infection. Sci. Hortic. 2013, 164, 461–465. [Google Scholar] [CrossRef]
- Smetanska, I.; Krumbein, A.; Schreiner, M.; Knorr, D. Influence of salicylic acid and methyl jasmonate on glucosinolate levels in turnip. J. Hortic. Sci. Biotechnol. 2007, 82, 690–694. [Google Scholar]
- Kiddle, G.A.; Doughty, K.J.; Wallsgrove, R.M. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J. Exp. Bot. 1994, 45, 1343–1346. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Knorr, D.; Smetanska, I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011, 59, 1400–1405. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J.; Sánchez-Martínez, M.J.; Pérez-Corona, M.T.; Madrid, Y.; Cámara, C.; Vidal-Valverde, C. Se improves indole glucosinolate hydrolysis products content, se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem. 2012, 132, 907–914. [Google Scholar] [CrossRef]
- Xu, F.; Chen, X.; Jin, P.; Wang, X.; Wang, J.; Zheng, Y. Effect of ethanol treatment on quality and antioxidant activity in postharvest broccoli florets. Eur. Food Res. Technol. 2012, 235, 793–800. [Google Scholar] [CrossRef]
- Kim, H.S.; Juvik, J.A. Effect of selenium fertilization and methyl jasmonate treatment on glucosinolate accumulation in broccoli florets. J. Am. Soc. Hortic. Sci. 2011, 136, 239–246. [Google Scholar]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Pérez, C.P.; Ulrichs, C.; Huyskens-Keil, S.; Schreiner, M.; Krumbein, A.; Schwarz, D.; Kläring, H.P. Composition of carotenoids in tomato fuits as affected by moderate UV-B radiation before harvest. ISHS Acta Hortic. 2009, 821, 217–222. [Google Scholar]
- Wang, C.Q.; Chen, M.; Wang, B.S. Betacyanin accumulation in the leaves of C3 halophyte suaeda salsa L. is induced by watering roots with H2O2. Plant Sci. 2007, 172, 1–7. [Google Scholar] [CrossRef]
- Trejo-Tapia, G.; Jimenez-Aparicio, A.; Rodriguez-Monroy, M.; de Jesus-Sanchez, A.; Gutierrez-Lopez, G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of of Beta vulgaris. Plant Cell Tiss. Org. 2001, 67, 19–23. [Google Scholar] [CrossRef]
- Gerster, H. Vitamin A functions, dietary requirements and safety in humans. Int. J. Vitam. Nutr. Res. 1997, 67, 71–90. [Google Scholar]
- Hoppe, P.P.; Krennrich, G. Bioavailability and potency of natural-source and all-racemic α-tocopherol in the human: A dispute. Eur.J. Nutr. 2000, 39, 183–193. [Google Scholar] [CrossRef]
- Puthusseri, B.; Divya, P.; Lokesh, V.; Neelwarne, B. Enhancement of folate content and its stability using food grade elicitors in coriander (Coriandrum sativum L.). Plant Food. Hum. Nutr. 2012, 67, 162–170. [Google Scholar] [CrossRef]
- Larsson, S.C.; Hakansson, N.; Giovannucci, E.; Wolk, A. Folate intake and pancreatic cancer incidence: A prospective study of swedish women and men. J. Natl. Cancer Inst. 2006, 98, 407–413. [Google Scholar] [CrossRef]
- Harris, J.R. Subcellular biochemistry, ascorbic acid: Biochemistry and biomedical cell biology, 25plenum, New York. Am. J. Clin. Nutr. 1996, 64, 830–831. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- wieca, M.; Surdyka, M.; Gawlik-Dziki, U.; Złotek, U.; Baraniak, B. Antioxidant potential of fresh and stored lentil sprouts affected by elicitation with temperature stresses. Int. J. Food Sci. Technol. 2014, 49, 1811–1817. [Google Scholar] [CrossRef]
- wieca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar]
- wieca, M.; Baraniak, B. Influence of elicitation with H2O2 on phenolics content, antioxidant potential and nutritional quality of lens culinaris sprouts. J. Sci. Food Agric. 2014, 94, 489–496. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Barona, G.; Ryan, C.A.; Pearce, G. GmPep914, an eight-amino acid peptide isolated from soybean leaves, activates defense-related genes. Plant Physiol. 2011, 156, 932–942. [Google Scholar] [CrossRef]
- Syeed, S.; Anjum, N.; Nazar, R.; Iqbal, N.; Masood, A.; Khan, N. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011, 33, 877–886. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Product Development under the Animal Rule; Division of Drug Information. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf?source=govdelivery&utm_medium=email&utm_source=govdelivery (accessed on 29 August 2014).
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541-13563. https://doi.org/10.3390/molecules190913541
Baenas N, García-Viguera C, Moreno DA. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules. 2014; 19(9):13541-13563. https://doi.org/10.3390/molecules190913541
Chicago/Turabian StyleBaenas, Nieves, Cristina García-Viguera, and Diego A. Moreno. 2014. "Elicitation: A Tool for Enriching the Bioactive Composition of Foods" Molecules 19, no. 9: 13541-13563. https://doi.org/10.3390/molecules190913541