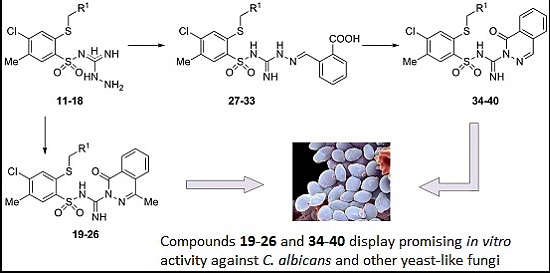
3.2. Synthesis
3.2.1. General Procedure for the Preparation of N-(2-Alkylthio-4-chloro-5-methylbenzenesulfonyl)-cyanamide Potassium Salts 8–10
A mixture of 2 (3 mmol) and the appropriate alkyl chloride (3.3 mmol) in water (9 mL) was stirred at 0 °C for 1–4 h. The solid was filtered off and crystallized from ethanol. In this manner the following potassium salts were obtained.
N-[4-Chloro-5-methyl-2-(4-methylbenzylthio)benzenesulfonyl]cyanamide potassium salt (8). Starting from 2 (1.017 g) and 4-methylbenzyl chloride (0.44 mL) in water with stirring for 1 h, 1.125 g (92%) of the title compound 8 was obtained, mp 198–200 °C; IR (KBr) νmax 2922 (C-H), 2174 (C≡N), 1343, 1142 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 2.32 (s, 3H, CH3), 4.2 (s, 2H, SCH2), 7.12–7.16 (d, J = 8.0 Hz, 2H, arom.), 7.3–7.34 (d, J = 7.9 Hz, 2H, arom.), 7.38 (s, 1H, H-3), 7.74 (s, 1H, H-6); anal. C 47.28, H 3.33, N 6.75% calcd for C16H14ClKN2O2S2, C 47.45, H 3.48, N 6.93%.
N-[4-Chloro-2-(4-chlorobenzylthio)-5-methylbenzenesulfonyl]cyanamide potassium salt (9). Starting from 2 (1.017 g) and 4-chlorobenzyl chloride (0.531 g) in water with stirring for 4 h, 1.253 g (98%) of the title compound 9 was obtained, mp 231–233 °C; IR (KBr) νmax 2920 (C-H), 2175 (C≡N), 1342, 1143 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.3 (s, 3H, CH3), 4.28 (s, 2H, SCH2), 7.34–7.50 (m, 5H, arom.), 7.73 (s, 1H, H-6); anal. C 42.33, H 2.58, N 6.55% calcd for C15H11Cl2KN2O2S2, C 42.35, H 2.61, N 6.59%.
N-{4-Chloro-5-methyl-2-[(2-phenylsulfonyl)ethylthio]benzenesulfonyl}cyanamide potassium salt (10). Starting from 2 (1.017 g) and 2-(phenylsulfonyl)ethyl chloride (0.675 g) in water with stirring for 1.5 h, 1.346 g (87%) of the title compound 10 was obtained, mp 126–129 °C; IR (KBr) νmax 2922 (C-H), 2179 (C≡N), 1343, 1151 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.31 (s, 3H, CH3), 3.12–3.19 (m, 2H, CH2S), 3.35–3.62 (m, 2H, CH2SO2), 7.26 (s, 1H, H-3), 7.63–7.7.81 (m, 4H, arom.), 7.92–7.96 (m, 2H, arom.); anal. C 40.90, H 2.96, N 5.90% calcd for C16H14ClKN2O4S3, C 40.97, H 3.01, N 5.97%.
3.2.2. General Procedure for the Preparation of 1-Amino-2-(2-alkylthio-4-chloro-5-methylbenzene- sulfonyl)guanidines 12–18
To a suspension of the appropriate N-(2-alkylthio-4-chloro-5-methylbenzenesulfonyl)cyanamide potassium salt 4–10 (3 mmol) in dry toluene (2.5–22.5 mL) hydrazine monohydrochloride (3 mmol) was added. A reaction mixture was stirred at reflux for 3–10 h, and left overnight at 0 °C. The precipitate was filtered off and dried, then treated with water (40 mL). After vigorously stirring for 30 min the precipitate was collected by filtration and dried. In this manner the following aminoguanidines were obtained:
1-Amino-2-[4-chloro-5-methyl-2-(4-trifluoromethylbenzylthio)benzenesulfonyl]guanidine (12). From 4 (1.377 g) in dry toluene (3 mL) with stirring for 4.5 h, 0.750 g (55%) of the title compound 12 was obtained, mp 210–212 °C; IR (KBr) νmax 3482, 3332 (NH2, NH), 2959 (C-H), 1324, 1129 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 4.40 (s, 2H, SCH2), 4.52 (s, 2H, CNH2), 7.00 (s, 2H, NNH2), 7.41 (s, 1H, H-3), 7.63 (d, 2H, arom.), 7.68 (d, 2H, arom.), 7.80 (s, 1H, H-6); anal. C 42.41, H 3.53, N 12.32% calcd for C16H16ClF3N4O2S2, C 42.43, H 3.56, N 12.37%.
1-Amino-2-[4-chloro-5-methyl-2-(3-trifluoromethylbenzylthio)benzenesulfonyl]guanidine (13). From 5 (1.377 g) in dry toluene (9 mL) with stirring for 3 h, 1.214 g (89%) of the title compound 13 was obtained, mp 154–155 °C; IR (KBr) νmax 3437, 3333 (NH2, NH), 2924, 2854 (C-H), 1330, 1126 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.29 (s, 3H, CH3), 4.41 (s, 2H, SCH2), 4.51 (s, 2H, CNH2), 6.98 (s, 2H, NNH2), 7.42 (s, 1H, H-3), 7.56–7.77 (m, 4H, arom.), 7.82 (s, 1H, H-6), 8.44 (s, 1H, NH); anal. C 42.38, H 3.49, N 12.30%, calcd for C16H16ClF3N4O2S2, C 42.43, H 3.56, N 12.37%.
1-Amino-2-[4-chloro-2-(6-chlorobenzo[d][1,3]dioxol-5-ylmethylthio)-5-methylbenzenesulfonyl]-guanidine (14). From 6 (1.401 g) in dry toluene (2.5 mL) with stirring for 4 h, 1.167 g (84%) of the title compound 14 was obtained, mp 245–250 °C; IR (KBr) νmax 3476, 3366 (NH2, NH), 2957, 2905 (C-H), 1343, 1131 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.32 (s, 3H, CH3), 4.24 (s, 2H, SCH2), 4.49 (s, 2H, CNH2), 6.08 (s, 2H, OCH2O), 6.98 (s, 2H, NNH2), 7.08 (s, 1H, arom.), 7.12 (s, 1H, arom.), 7.38 (s, 1H, H-3), 7.84 (s, 1H, H-6), 8.43 (s, 1H, NH); anal. C 41.45, H 3.49, N 12.10% calcd for C16H16Cl2N4O4S2, C 41.47, H 3.48, N 12.09%.
1-Amino-2-[4-chloro-2-(2,3-dihydrobenzo[b][1,4]-dioxan-2-ylmethylthio)-5-methylbenzenesulfonyl]-guanidine (15). From 7 (1.347 g) in dry toluene (3 mL) with stirring for 4 h, 1.157 g (87%) of the title compound 15 was obtained, mp 171–175 °C; IR (KBr) νmax 3441, 3335, 3223 (NH2, NH), 2924, 2854 (C-H), 1344, 1132 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.30 (s, 3H, CH3), 3.30 (s, 2H, SCH2), 4.01–4.11 (m, 1H, CHO), 4.30–4.40 (m, 2H, CH2O), 4.50 (s, 2H, CNH2), 6.80 (s, 4H, arom.), 7.00 (s, 2H, NNH2), 7.60 (s, 1H, H-3), 7.80 (s, 1H, H-6), 8.40 (s, 1H, NH); anal. C 45.99, H 4.31, N 12.63% calcd for C17H19ClN4O4S2, C 46.10, H 4.32, N 12.65%.
1-Amino-2-[4-chloro-5-methyl-2-(4-methylbenzylthio)benzenesulfonyl]guanidine (16). From 8 (1.215 g) in dry toluene (2.5 mL) with stirring for 4 h, 0.965 g (81%) of the title compound 16 was obtained, mp 200–202 °C; IR (KBr) νmax 3472, 3364, 3342 (NH2, NH), 2958, 2924 (C-H), 1339, 1135 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.25 (s, 3H, CH3), 2.35 (s, 3H, CH3), 4.25 (s, 2H, SCH2), 4.50 (s, 2H, CNH2), 7.00 (s, 2H, NNH2), 7.10–7.20 (d, J = 8.0 Hz, 2H, arom.), 7.28–7.36 (d, J = 8.0 Hz, 2H, arom.), 7.42 (s, 1H, H-3), 7.80 (s, 1H, H-6), 8.40 (s, 1H, NH); anal. C 48.14, H 4.75, N 14.00% calcd for C16H19ClN4O2S2, C 48.17, H 4.80, N 14.04%.
1-Amino-2-[4-chloro-2-(4-chlorobenzylthio)-5-methylbenzenesulfonyl]guanidine (17). From 9 (1.277 g) in dry toluene (18 mL) with stirring for 10 h, 1.070 g (85%) of the title compound 17 was obtained, mp 200–203 °C; IR (KBr) νmax 3476, 3359 (NH2, NH), 2961, (C-H), 1342, 1127 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.29 (s, 3H, CH3), 4.30 (s, 2H, SCH2), 4.51 (s, 2H, CNH2), 6.98 (s, 2H, NNH2), 7.34–7.50 (m, 5H, arom.), 7.80 (s, 1H, H-6), 8.42 (s, 1H, NH); anal. C 42.92, H 3.82, N 13.34% calcd for C15H16Cl2N4O2S2, C 42.96, H 3.85, N 13.36%.
1-Amino-2-{4-chloro-5-methyl-2-[(2-phenylsulfonyl)ethylthio]benzenesulfonyl}guanidine (18). From 10 (1.407 g) in dry toluene (16.5 mL) with stirring for 4 h, 1.361 g (98%) of the title compound 18 was obtained, mp 183–185 °C; IR (KBr) νmax 3455, 3349 (NH2, NH), 2923, 2853 (C-H), 1314, 1144 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.30 (s, 3H, CH3), 3.10–3.25 (m, 2H, SCH2), 3.50–3.65 (m, 2H, CH2SO2), 4.50 (s, 2H, CNH2), 6.97 (s, 2H, NNH2), 7.30 (s, 1H, H-3), 7.62–7.86 (m, 4H, arom.), 7.90–8.00 (m, 2H, arom.), 8.40 (s, 1H, NH); anal. C 41.49, H 4.13, N 12.05% calcd for C16H19ClN4O4S3, C 41.51, H 4.14, N 12.10%.
3.2.3. General Procedure for the Preparation of 2-Alkylthio-4-chloro-5-methyl-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamides 19–26
To a suspension of the appropriate 1-amino-2-(2-alkylthio-4-chloro-5-methylbenzenesulfonyl)guanidine 11–18 (0.5 mmol) in glacial acetic acid (19–22, 24–26—1 mL) or 1,4-dioxane (23—2.2 mL) 2-acetylbenzoic acid (0.082 g, 0.5 mmol) was added. A reaction mixture was stirred at reflux for 1–8 h, the precipitate was filtered off, dried and crystallized from acetonitrile (19, 24, 26), ethanol (20–21, 23, 25) or benzene (22). In this manner the following compounds were obtained:
2-Benzylthio-4-chloro-5-methyl-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]benzene-sulfonamide (19). Starting from 11 (0.192 g) with stirring for 5 h, 0.110 g (51%) of the title compound 19 was obtained, mp 170–175 °C; IR (KBr) νmax 3330, 3210 (NH), 2923, 2853 (C-H), 1664 (C=O), 1638 (NH), 1343, 1145 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3Ph), 2.48 (s, 3H, CH3), 4.36 (s, 2H, SCH2), 7.25–7.35 (m, 3H, arom.), 7.44–7.48 (m, 2H, arom.), 7.51 (s, 1H, H-3), 7.77 (s, 1H, H-6), 7.91–8.05 (m, 3H, H-5, H-6, H-7 phthalazine), 8.29–8.33 (d, J = 7.8 Hz , 1H, H-8 phthalazine), 8.95 (s, 1H, C=NH), 9.29 (s, 1H, SO2NH); anal. C 56.17, H 4.16, N 11.05% calcd for C24H21ClN4O3S2, C 56.19, H 4.13, N 10.92%.
4-Chloro-5-methyl-2-(4-trifluoromethylbenzylthio)-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)-methyl]benzenesulfonamide (20). Starting from 12 (0.209 g) with stirring for 1 h, 0.160 g (53%) of the title compound 20 was obtained, mp 169–174 °C; IR (KBr) νmax 3331 (NH), 2923, 2853 (C-H), 1664 (C=O), 1642 (NH), 1528, 1447 (C=C, C=N), 1324, 1146 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.15 (s, 3H, CH3Ph), 2.41 (s, 3H, CH3), 4.44 (s, 2H, SCH2), 7.48 (s, 1H, H-3), 7.57–7.59 (d, J = 7.5 Hz, 2H, arom.), 7.63–7.65 (d, J = 7.5 Hz, 2H, arom.), 7.72 (s, 1H, H-6), 7.90–8.02 (m, 3H, H-5, H-6, H-7 phthalazine), 8.26–8.27 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.93 (s, 1H, C=NH), 9.29 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 18.4, 18.9, 35.9, 125.1, 126.1, 126.4, 126.9, 128.7, 129.2, 129.9, 130.8, 132.4, 132.5, 134.5, 135.4, 137.3, 138.4, 141.5, 144.2, 154.4, 157.2; anal. C 51.66, H 3.48, N 9.65% calcd for C25H20ClF3N4O3S2, C 51.68, H 3.47, N 9.64%.
4-Chloro-5-methyl-2-(3-trifluoromethylbenzylthio)-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (21). Starting from 13 (0.209 g) with stirring for 1.5 h, 0.125 g (43%) of the title compound 21 was obtained, mp 145–149 °C; IR (KBr) νmax 3333, 3208 (NH), 2923 (C-H), 1665 (C=O), 1639 (NH), 1526, 1449 (C=C, C=N), 1331, 1145 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.16 (s, 3H, CH3Ph), 2.42 (s, 3H, CH3), 4.44 (s, 2H, SCH2), 7.47–7.50 (m, 2H, H-3, arom.), 7.57–7.59 (m, 2H, arom.), 7.76–7.78 (m, 3H, H-6, arom.), 7.89–8.01 (m, 3H, H-5, H-6, H-7 phthalazine), 8.25–8.27 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.93 (s, 1H, C=NH), 9.28 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 19.0, 19.5, 36.7, 124.6, 124.7, 126.5, 126.8, 127.1, 127.6, 129.7, 129.8, 129.9, 130.2, 131.5, 133.1, 133.3, 133.9, 135.1, 136.0, 137.9, 138.7, 139.1, 144.9, 155.1, 157.9; anal. C 51.63, H 3.44, N 9.59% calcd for C25H20ClF3N4O3S2, C 51.68, H 3.47, N 9.64%.
4-Chloro-2-(6-chlorobenzo[d][1,3]dioxol-5-ylmethylthio)-5-methyl-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (22). Starting from 14 (0.231 g) with stirring for 8 h, 0.127 g (43%) of the title compound 22 was obtained, mp 159–162 °C; IR (KBr) νmax 3414, 3333 (NH), 2920 (C-H), 1662 (C=O), 1639 (NH), 1527, 1804 (C=C, C=N), 1345, 1145 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3Ph), 2.47 (s, 3H, CH3), 4.30 (s, 2H, SCH2), 6.01 (s, 2H, OCH2O), 7.07 (s, 1H, arom.), 7.10 (s, 1H, arom.), 7.44 (s, 1H, H-3), 7.78 (s, 1H, H-6), 7.92–8.03 (m, 3H, H-5, H-6, H-7 phthalazine), 8.26–8.30 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.90 (s, 1H, C=NH), 9.35 (s, 1H, SO2NH); anal. C 50.73, H 3.40, N 9.45% calcd for C25H20Cl2N4O5S2, C 50.76, H 3.41, N 9.47%.
4-Chloro-2-(2,3-dihydrobenzo[b][1,4]-dioxan-2-ylmethylthio)-5-methyl-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (23). Starting from 15 (0.222 g) with stirring for 5 h, 0.126 g (44%) of the title compound 23 was obtained, mp 156–160 °C; IR (KBr) νmax 3331, 3211 (NH), 2923, 2847 (C-H), 1666 (C=O), 1637 (NH), 1523, 1494 (C=C, C=N), 1343, 1145 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3Ph), 2.47 (s, 3H, CH3), 3.46–3.49 (m, 2H, SCH2), 4.04–4.10 (m, 1H, OCH), 4.35–4.39 (m, 2H, OCH2), 6.71–6.81 (m, 4H, arom.), 7.74 (s, 1H, H-3), 7.78 (s, 1H, H-6), 7.92–8.03 (m, 3H, H-5, H-6, H-7 phthalazine), 8.23–8.27 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.94 (s, 1H, C=NH), 9.30 (s, 1H, SO2NH); anal. C 54.63, H 4.03, N 9.79% calcd for C26H23ClN4O5S2, C 54.68, H 4.06, N 9.81%.
4-Chloro-5-methyl-2-(4-methylbenzylthio)-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]-benzenesulfonamide (24). Starting from 16 (0.200 g) with stirring for 6 h, 0.081 g (30%) of the title compound 24 was obtained, mp 174–180 °C; IR (KBr) νmax 3372, 3231 (NH), 2921, 2853 (C-H), 1685 (C=O), 1634 (NH), 1575, 1456 (C=C, C=N), 1344, 1145 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.17 (s, 3H, CH3Ph), 2.22 (s, 3H, CH3Ph), 2.45 (s, 3H, CH3), 4.27 (s, 2H, SCH2), 7.04–7.06 (d, J = 7.9 Hz, 2H, arom.), 7.29–7.30 (d, J = 7.9 Hz, 2H, arom.), 7.47 (s, 1H, H-3), 7.73 (s, 1H, H-6), 7.90–8.02 (m, 3H, H-5, H-6, H-7 phthalazine), 8.26–8.28 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.89 (s, 1H, C=NH), 9.26 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 19.1, 19.5, 21.4, 37.1, 126.8, 127.1, 127.6, 128.9, 129.6, 129.8, 129.9, 131.4, 132.7, 133.1, 133.7, 135.1, 137.1, 137.2, 137.9, 138.5, 144.9, 155.0, 157.9; anal. C 56.95, H 4.36, N 10.67% calcd for C25H23ClN4O3S2, C 56.97, H 4.40, N 10.63%.
4-Chloro-2-(4-chlorobenzylthio)-5-methyl-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)methyl]-benzenesulfonamide (25). Starting from 17 (0.210 g) with stirring for 1 h, 0.160 g (53%) of the title compound 25 was obtained, mp 169–174 °C; IR (KBr) νmax 3230 (NH), 2920 (C-H), 1683 (C=O), 1634 (NH), 1575, 1487 (C=C, C=N), 1344, 1144 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.16 (s, 3H, CH3Ph), 2.43 (s, 3H, CH3), 4.33 (s, 2H, SCH2), 7.29–7.30 (d, J = 7.8 Hz, 2H, arom.), 7.43–7.47 (m, 3H, H-3, arom.), 7.72 (s, 1H, H-6), 7.90–8.02 (m, 3H, H-5, H-6, H-7 phthalazine), 8.26–8.28 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.92 (s, 1H, C=NH), 9.27 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 19.1, 19.5, 36.5, 126.8, 127.1, 127.5, 129.0, 129.3, 129.9, 131.4, 131.7, 132.5, 133.0, 133.2, 135.1, 136.1, 136.5, 137.9, 138.9, 144.9, 155.0, 157.9; anal. C 52.67, H 3.66, N 10.26% calcd for C24H20Cl2N4O3S2, C 52.65, H 3.68, N 10.23%.
4-Chloro-5-methyl-2-[(2-phenylsulfonyl)ethylthio]-N-[imino-(4-methyl-1-oxo-(1H)-phthalazin-2-yl)-methyl]benzenesulfonamide (26). Starting from 18 (0.238 g) with stirring for 3.5 h, 0.190 g (64%) of the title compound 25 was obtained, mp 193–195 °C; IR (KBr) νmax 3372, 3231 (NH), 2921, 2853 (C-H), 1685 (C=O), 1634 (NH), 1575, 1456 (C=C, C=N), 1344, 1145 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.13 (s, 3H, CH3Ph), 2.45 (s, 3H, CH3), 3.20–3.27 (m, 2H, CH2), 3.55–3.63 (m, 2H, CH2), 7.45 (s, 1H, H-3), 7.61–7.81 (m, 4H, arom.), 7.89–8.06 (m, 5H, H-5, H-6 and H-7 phthalazine, arom.), 8.16–8.20 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.91 (s, 1H, C=NH), 9.25 (s, 1H, SO2NH); 13C-NMR (50 MHz, DMSO-d6) δ 18.7, 19.6, 26.4, 54.3, 126.4, 126.6, 127.1, 128.0,129.4, 129.9, 130.0, 131.1, 132.7, 133.8, 134.3, 134.4, 134.7, 137.9, 138.6, 139.8, 144.6, 154.6, 157.3; anal. C 50.82, H 3.91, N 9.51% calcd for C25H23ClN4O5S3, C 50.80, H 3.92, N 9.48%.
3.2.4. General Procedure for the Preparation of N-(2-Alkylthio-4-chloro-5-methylbenzenesulfonyl)-2-(2-carboxybenzylidene)hydrazinecarboximidamides 27–33
To a suspension of the appropriate 1-amino-2-(2-alkylthio-4-chloro-5-methylbenzenesulfonyl)guanidine (11–17) (1.5 mmol) in glacial acetic acid (3 mL) 2-formylbenzoic acid (0.225 g, 1.5 mmol) was added. A reaction mixture was stirred at reflux for 1–11 h, the precipitate was filtered off and dried. In this manner the following compounds were obtained:
N-(2-Benzylthio-4-chloro-5-methylbenzenesulfonyl)-2-(2-carboxybenzylidene)hydrazinecarboximid-amide (27). Starting from 11 (0.576 g) with stirring for 11 h, 0.348 g (45%) of the title compound 27 was obtained, mp 205–207 °C; IR (KBr) νmax 3456, 3345 (NH), 2923 (C-H), 1674 (C=O), 1626 (NH), 1599, 1485 (C=C, C=N), 1345, 1139 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.35 (s, 3H, CH3Ph), 4.38 (s, 2H, SCH2), 7.19–7.29 (m, 4H, C=NH, arom.), 7.39–7.45 (m, 2H, N=CH, arom.), 7.50–7.62 (m, 3H, arom.), 7.80 (s, 1H, H-3), 7.86–7.88 (m, 1H, arom.), 7.93 (brs, 1H, NHN), 8.26–8.28 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.83 (s, 1H, H-6), 11.56 (s, 1H, SO2NH), 13.35 (brs, 1H, COOH); anal. C 53.39, H 4.11, N 10.86% calcd for C23H21ClN4O4S2, C 53.43, H 4.09, N 10.84%.
2-(2-Carboxybenzylidene)-N-[2-(4-trifluoromethylbenzylthio)-4-chloro-5-methylbenzenesulfonyl]-hydrazinecarboximidamide (28). Starting from 12 (0.627 g) with stirring for 1.5 h, 0.684 g (78%) of the title compound 28 was obtained, mp 203–208 °C; IR (KBr) νmax 3452, 3335 (NH), 2923 (C-H), 1670 (C=O), 1630 (NH), 1565, 1486 (C=C, C=N), 1323, 1140 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.33 (s, 3H, CH3Ph), 4.46 (s, 2H, SCH2), 7.24 (brs, 1H, C=NH),7.48–7.67 (m, 7H, N=CH, arom.), 7.82–7.97 (m, 3H, NHN, arom.), 8.26–8.30 (d, J = 7.4 Hz, 1H, H-3 carboxy-benzylidene), 8.87 (s, 1H, H-6), 11.58 (s, 1H, SO2NH), 13.35 (brs, 1H, COOH); anal. C 49.30, H 3.48, N 9.57% calcd for C24H20ClF3N4O4S2, C 49.27, H 3.45, N 9.58%.
2-(2-Carboxybenzylidene)-N-[2-(3-trifluoromethylbenzylthio)-4-chloro-5-methylbeneznesulfonyl]-hydrazinecarboximidamide (29). Starting from 13 (0.627 g) with stirring for 1.5 h, 0.639 g (73%) of the title compound 29 was obtained, mp 187–191 °C; IR (KBr) νmax 3454, 3348 (NH), 2922 (C-H), 1663 (C=O), 1625 (NH), 1569, 1486 (C=C, C=N), 1329, 1136 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.32 (s, 3H, CH3Ph), 4.47 (s, 2H, SCH2), 7.25 (brs, 1H, C=NH),7.47–7.64 (m, 5H, N=CH, arom.), 7.71–7.78 (m, 2H, arom.), 7.86–7.93 (m, 3H, NHN, arom.), 8.24–8.28 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.83 (s, 1H, H-6), 11.55 (s, 1H, SO2NH), 13.35 (brs, 1H, COOH); anal. C 49.25, H 3.12, N 9.54% calcd for C24H20ClF3N4O4S2, C 49.27, H 3.45, N 9.58%.
2-(2-Carboxybenzylidene)-N-[2-(6-chlorobenzo[d][1,3]dioxol-5-ylmethylthio)-4-chloro-5-methyl-beneznesulfonyl]hydrazinecarboximidamide (30). Starting from 14 (0.693 g) with stirring for 7 h, 0.804 g (90%) of the title compound 30 was obtained, mp 211–213 °C; IR (KBr) νmax 3449, 3342 (NH), 2922 (C-H), 1672 (C=O), 1626 (NH), 1598, 1487 (C=C, C=N), 1360, 1136 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.36 (s, 3H, CH3Ph), 4.29 (s, 2H, SCH2), 5.98 (s, 2H, OCH2O), 7.25 (brs, 1H, C=NH),7.49–7.65 (m, 3H, N=CH, arom.), 7.79 (brs, 1H, NHN), 7.86–7.90 (d, J = 7.6 Hz, 1H, arom.), 7.96 (s, 1H, H-3), 8.25–8.29 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.82 (s, 1H, H-6), 11.52 (s, 1H, SO2NH); anal. C 48.45, H 3.38, N 9.46% calcd for C24H20Cl2N4O6S2, C 48.41, H 3.39, N 9.41%.
2-(2-Carboxybenzylidene)-N-[2-(2,3-dihydrobenzo[b][1,4]-dioxan-2-ylmethylthio)-4-chloro-5-methyl-beneznesulfonyl]hydrazinecarboximidamide (31). Starting from 15 (0.666 g) with stirring for 1 h, 0.639 g (74%) the title compound 31 was obtained, mp 196–202 °C; IR (KBr) νmax 3457, 3347 (NH), 2922 (C-H), 1666 (C=O), 1625 (NH), 1596, 1444 (C=C, C=N), 1345, 1140 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.37 (s, 3H, CH3Ph), 3.36–3.42 (m, 2H, SCH2), 4.01–4.11 (m, 1H, CHO), 4.34–4.39 (m, 2H, CH2O), 6-70-6.80 (m, 4H, arom.), 7.29 (brs, 1H, C=NH),7.48–7.66 (m, 3H, N=CH, arom.), 7.86–7.89 (m, 2H, NHN, arom.), 7.98 (s, 1H, H-3), 8.26–8.30 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.83 (s, 1H, H-6), 11.56 (s, 1H, SO2NH), 13.10 (brs, 1H, COOH); anal. C 52.19, H 3.98, N 9.76% calcd for C25H23ClN4O6S2, C 52.22, H 4.03, N 9.74%.
2-(2-Carboxybenzylidene)-N-[2-(4-methylbenzylthio)-4-chloro-5-methylbeneznesulfonyl]hydrazine-carboximidamide (32). Starting from 16 (0.600 g) with stirring for 11 h, 0.726 g (91%) of the title compound 32 was obtained, mp 204–210 °C; IR (KBr) νmax 3456, 3344 (NH), 2922 (C-H), 1674 (C=O), 1627 (NH), 1598, 1485 (C=C, C=N), 1346, 1141 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.15 (s, 3H, CH3Ph), 2.30 (s, 3H, CH3Ph), 4.25 (s, 2H, SCH2), 7.02–7.03 (m, 2H, arom.), 7.21 (s, 1H, C=NH), 7.25–7.27 (m, 2H, arom.), 7.49–7.59 (m, 3H, N=CH, arom.), 7.77 (s, 1H, NHN), 7.85 (m, 1H, arom.), 7.89 (s, 1H, H-3), 8.25–8.26 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.83 (s, 1H, H-6), 11.53 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 19.6, 21.3, 36.6, 127.8, 128.2, 129.6, 129.7, 130.3, 130.8, 131.1, 131.3, 132.4, 132.5, 133.7, 134.6, 136.4, 136.9, 137.2, 140.0, 144.2, 155.7, 168.8; anal. C 54.30, H 4.35, N 10.58% calcd for C24H23ClN4O4S2, C 54.28, H 4.37, N 10.55%.
2-(2-Carboxybenzylidene)-N-[2-(4-chlorobenzylthio)-4-chloro-5-methylbeneznesulfonyl]hydrazine-carboximidamide (33). Starting from 17 (0.630 g) with stirring for 3 h, 0.786 g (95%) of the title compound 33 was obtained, mp 184–189 °C; IR (KBr) νmax 3456, 3342 (NH), 2920 (C-H), 1672 (C=O), 1627 (NH), 1563, 1486 (C=C, C=N), 1345, 1141 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.33 (s, 3H, CH3Ph), 4.36 (s, 2H, SCH2), 7.24 (brs, 1H, C=NH), 7.28–7.32 (d, J = 8.4 Hz, 2H, arom.), 7.41–7.46 (d, J = 8.4 Hz, 2H, arom.), 7.53–7.65 (m, 3H, N=CH, arom.), 7.82–7.93 (m, 3H, NH-N, H-3, arom.), 8.26–8.36 (d, J = 7.4 Hz, 1H, H-3 carboxybenzylidene), 8.86 (s, 1H, H-6), 11.57 (s, 1H, SO2NH), 13.28 (brs, 1H, COOH); anal. C 50.05, H 3.59, N 10.05% calcd for C23H20Cl2N4O4S2, C 50.09, H 3.66, N 10.16%.
3.2.5. General Procedure for the Preparation of 2-Alkylthio-4-chloro-5-methyl-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamides 34–40
To a suspension of the appropriate N-(2-alkylthio-4-chloro-5-methylbeneznesulfonyl)-2-(2-carboxybenzylidene)hydrazinecarboximidamide (27–33) (0.5 mmol) in toluene (5 mL) p-toluenesulfonic acid (0.026 g, 0.15 mmol) was added. A reaction mixture was stirred at reflux for 1–4.5 h, the precipitate was filtered off, dried and crystallized from ethanol (34, 36) acetonitrile (35, 37, 39–40) or p-dioxane (38). In this manner the following compounds were obtained:
2-Benzylthio-4-chloro-5-methyl-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (34). Starting from 27 (0.258 g) with stirring for 3 h, 0.160 g (64%) of the title compound 34 was obtained, mp 166–170 °C; IR (KBr) νmax 3314, 3199 (NH), 2920 (C-H), 1670 (C=O), 1642 (NH), 1316, 1147 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3Ph), 4.32 (s, 2H, SCH2), 7.22–7.28 (m, 3H, arom.), 7.42–7.43 (m, 2H, arom.), 7.47 (s, 1H, H-3), 7.79 (s, 1H, H-4 phthalazine), 7.91–7.99 (m, 3H, H-5, H-6, H-7 phthalazine), 8.24–8.25 (d, J = 7.8 Hz , 1H, H-8 phthalazine), 8.48 (s, 1H, H-6), 8.89 (s, 1H, C=NH), 9.33 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ b19.5, 37.3, 126.7, 127.8, 127.9, 128.1, 129.0, 129.1, 129.8, 129.9, 131.5, 132.8, 133.5, 135.3, 136.8, 137.1, 138.0, 138.3, 139.5, 155.0, 158.0; anal. 55.39, H 3.82, N 11.20% calcd for C23H19ClN4O3S2, C 55.36, H 3.84, N 11.23%.
4-Chloro-5-methyl-2-(4-trifluoromethylbenzylthio)-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]-benzenesulfonamide (35). Starting from 28 (0.293 g) with stirring for 2 h, 0.122 g (43%) of the title compound 35 was obtained, mp 162–167 °C; IR (KBr) νmax 3372 (NH), 2922 (C-H), 1677 (C=O), 1629 (NH), 1554, 1453 (C=C, C=N), 1323, 1141 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3Ph), 4.44 (s, 2H, SCH2), 7.49 (s, 1H, H-3), 7.58–7.60 (d, J = 8.0 Hz, 2H, arom.), 7.64–7.65 (d, J = 8.0 Hz, 2H, arom.), 7.80 (s, 1H, H-4 phthalazine), 7.91–8.00 (m, 3H, H-5, H-6, H-7 phthalazine), 8.23–8.25 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.49 (s, 1H, H-6), 8.90 (s, 1H, C=NH), 9.35 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ 19.5, 36.6, 125.8, 125.9, 126.7, 127.8, 128.1, 129.4, 129.9, 130.6, 131.5, 133.3, 133.5, 135.3, 136.1, 138.1, 138.7, 139.6, 142.2, 155.1, 158.0; anal. C 50.80, H 3.15, N 9.85% calcd for C24H18ClF3N4O3S2, C 50.84, H 3.20, N 9.88%.
4-Chloro-5-methyl-2-(3-trifluoromethylbenzylthio)-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]-benzenesulfonamide (36). Starting from 29 (0.293 g) with stirring for 1 h, 0.057 g (20%) of the title compound 36 was obtained, mp 158–163 °C; IR (KBr) νmax 3389 (NH), 2923 (C-H), 1672 (C=O), 1640 (NH), 1520, 1451 (C=C, C=N), 1330, 1116 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.22 (s, 3H, CH3Ph), 4.47 (s, 2H, SCH2), 7.50 (s, 1H, H-3), 7.55–7.62 (m, 2H, arom.), 7.74–7.78 (m, 2H, arom.), 7.82 (s, 1H, H-4 phthalazine), 7.89–8.01 (m, 3H, H-5, H-6, H-7 phthalazine), 8.24–8.28 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.48 (s, 1H, H-6), 8.90 (s, 1H, C=NH), 9.35 (s, 1H, SO2NH); anal. C 50.87, H 3.24, N 9.89% calcd for C24H18ClF3N4O3S2, C 50.84, H 3.20, N 9.88%.
4-Chloro-2-(6-chlorobenzo[d][1,3]dioxol-5-ylmethylthio)-5-methyl-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (37). Starting from 30 (0.298 g) with stirring for 2 h, 0.101 g (35%) of the title compound 37 was obtained, mp 180–185 °C; IR (KBr) νmax 3363, 3236 (NH), 2923, 2853 (C-H), 1676 (C=O), 1633 (NH), 1532, 1480 (C=C, C=N), 1344, 1138 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.25 (s, 3H, CH3Ph), 4.31 (s, 2H, SCH2), 6.02 (s, 2H, OCH2O), 7.07 (s, 1H, arom.), 7.12 (s, 1H, arom.), 7.45 (s, 1H, H-3), 7.85 (s, 1H, H-4 phthalazine), 7.94–8.04 (m, 3H, H-5, H-6, H-7 phthalazine), 8.25–8.28 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.48 (s, 1H, H-6), 8.90 (s, 1H, C=NH), 9.35 (s, 1H, SO2NH); anal. C 49.90, H 3.15, N 9.68% calcd for C25H20Cl2N4O5S2, C 49.92, H 3.14, N 9.70%.
4-Chloro-2-(2,3-dihydrobenzo[b][1,4]-dioxan-2-ylmethylthio)-5-methyl-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzenesulfonamide (38). Starting from 31 (0.288 g) with stirring for 2 h, 0.175 g (63%) of the title compound 38 was obtained, mp 195–199 °C; IR (KBr) νmax 3380 (NH), 2920 (C-H), 1669 (C=O), 1636 (NH), 1526, 1491 (C=C, C=N), 1344, 1135 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.23 (s, 3H, CH3Ph), 3.46–3.49 (m, 2H, SCH2), 4.05–4.10 (m, 1H, OCH), 4.35–4.40 (m, 2H, OCH2), 6.73–6.81 (m, 4H, arom.), 7.74 (s, 1H, H-3), 7.84 (s, 1H, H-4 phthalazine), 7.89–8.02 (m, 3H, H-5, H-6, H-7 phthalazine), 8.20–8.24 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.45 (s, 1H, H-6), 8.94 (s, 1H, C=NH), 9.39 (s, 1H, SO2NH); anal. C 53.87, H 3.81, N 10.03% calcd for C25H21ClN4O5S2, C 53.90, H 3.80, N 10.06%.
4-Chloro-5-methyl-2-(4-methylbenzylthio)-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzene-sulfonamide (39). Starting from 32 (0.114 g) with stirring for 4.5 h, 0.164 g (64%) of the title compound 39 was obtained, mp 184–186 °C; IR (KBr) νmax 3364, 3227 (NH), 2920, 2854 (C-H), 1681 (C=O), 1630 (NH), 1556, 1453 (C=C, C=N), 1339, 114 (SO2) cm−1; 1H-NMR (200 MHz, DMSO-d6) δ 2.24 (s, 3H, CH3Ph), 2.26 (s, 3H, CH3Ph), 4.31 (s, 2H, SCH2), 7.06–7.11 (d, J = 8.0 Hz, 2H, arom.), 7.31–7.35 (d, J = 8.0 Hz, 2H, arom.), 7.51 (s, 1H, H-3), 7.82 (s, 1H, H-4 phthalazine), 7.91–8.05 (m, 3H, H-5, H-6, H-7 phthalazine), 8.26–8.30 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.52 (s, 1H, H-6), 8.90 (s, 1H, C=NH), 9.35 (s, 1H, SO2NH); anal. C 56.25, H 4.12, N 11.00% calcd for C24H21ClN4O3S2, C 56.19, H 4.13, N 10.92%.
4-Chloro-2-(4-chlorobenzylthio)-5-methyl-N-[imino-(1-oxo-(1H)-phthalazin-2-yl)methyl]benzene-sulfonamide (40). Starting from 33 (0.276 g) with stirring for 1.5 h, 0.100 g (37%) of the title compound 40 was obtained, mp 164–168 °C; IR (KBr) νmax 3363, 3229 (NH), 2921 (C-H), 1679 (C=O), 1630 (NH), 1557, 1489 (C=C, C=N), 1337, 1142 (SO2) cm−1; 1H-NMR (500 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3Ph), 4.34 (s, 2H, SCH2), 7.30–7.31 (d, J = 8.0 Hz, 2H, arom.), 7.44–7.47 (m, 3H, H-3, arom.), 7.79 (s, 1H, H-4 phthalazine), 7.92–8.00 (m, 3H, H-5, H-6, H-7 phthalazine), 8.24–8.25 (d, J = 7.8 Hz, 1H, H-8 phthalazine), 8.49 (s, 1H, H-6), 8.89 (s, 1H, C=NH), 9.33 (s, 1H, SO2NH); 13C-NMR (125 MHz, DMSO-d6) δ b19.5, 36.4, 126.7, 127.8, 128.1, 129.0, 129.3, 129.9, 131.5, 131.6, 132.5, 133.1, 133.5, 135.3, 136.1, 136.5, 138.0, 138.6, 139.6, 155.1, 158.0; anal. C 51.77, H 3.36, N 10.50% calcd for C23H18Cl2N4O3S2, C 51.78, H 3.40, N 10.50%.