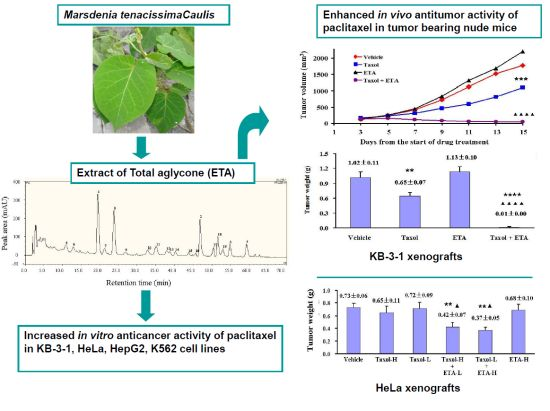
Total Aglycones from Marsdenia tenacissima Increases Antitumor Efficacy of Paclitaxel in Nude Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enhanced Inhibitory Effect of Paclitaxel on Cell Viability by ETA
| Treatment | Tumor Cell Lines | |||
|---|---|---|---|---|
| K562 | HeLa | HepG2 | KB-3-1 | |
| Paclitaxel alone (Control) | 4.8 ± 0.4 | 17.1 ± 4.1 | 67.2 ± 12.3 | 8.1 ± 1.3 |
| Paclitaxel + 10 μg/mL ETA | 3.8 ± 0.6 | 8.3 ± 1.0 * | 14.1 ± 8.5 ** | 5.1 ± 0.6 * |
2.2. Enhanced Inhibitory Effect of Paclitaxel on Tumor Growth in Nude Mice by ETA


2.3. Chemical Composition of ETA

2.4. Assay of 1
3. Experimental Section
3.1. Preparation of the Extract of Total Aglycones of M. tenacissima
3.2. In Vitro Anticancer Activity of Paclitaxel in the Presence or Absence of ETA
3.3. In Vivo Anti-Tumor Effect of Paclitaxel in the Presence or Absence of ETA
3.4. Statistical Analysis
3.5. Chemical Compositional Analysis of ETA
3.6. Assay of Marker Compound 1
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, J.; Chen, W.Q. Chinese Cancer Registry Annual Report; Military Medical Science Press: Beijing, China, 2012. [Google Scholar]
- Ozben, T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006, 580, 2903–2909. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Johnson, I.S.; Armstrong, J.G.; Gorman, M.; Burnett, J.P., Jr. The vinca alkaloids: A new class of oncolytic agents. Cancer Res. 1963, 23, 1390–1427. [Google Scholar]
- Wani, M.; Taylor, H.; Wall, M.; Coggon, P.; McPhail, A. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Adams, D.J.; Wahl, M.L.; Flowers, J.L.; Sen, B.; Colvin, M.; Dewhirst, M.W.; Manikumar, G.; Wani, M.C. Camptothecin analogs with enhanced activity against human breast cancer cells. II. Impact of the tumor pH gradient. Cancer Chemother. Pharmacol. 2005, 57, 145–154. [Google Scholar]
- Editorial Committee of the Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. Part 1; Chinese Medical Science and Technology Press: Beijing, China, 2010; pp. 277–278. [Google Scholar]
- Drug Standards of the State Drug Administration of China [S]; The State Drug Administration of China: Beijing, China, 2002; Standard code: WS-10630 (ZD-0630)-2002.
- Yuan, X.Y.; Fang, Z.Z.; Huang, X.Y. Observation on the treatment of 14 cases of advanced gastric cancer with Injection Xiao-Ai-Ping. Shanghai Med. Pharm. J. 1996, 6, 12–13. [Google Scholar]
- Wang, W.Y.; Zhou, Y.; Zhang, X.J.; Gao, T.H.; Luo, Z.F.; Liu, M.Y. A random study of xiaoaiping injection combined with chemotherapy on the treatment of advanced non-small cell lung cancer. Chin. Clin. Oncol. 2009, 14, 936–938. [Google Scholar]
- Han, S.Y.; Zhao, M.B.; Zhuang, G.B.; Li, P.P. Marsdenia tenacissima extract restored gefitinib sensitivity in resistant non-small cell lung cancer cells. Lung Cancer 2012, 5, 30–37. [Google Scholar]
- Huang, T.; Gong, W.H.; Zou, C.P.; Li, X.C.; Jiang, G.J.; Li, X.H.; Qian, H. Marsdenia tenacissima extract sensitizes MG63 cells to doxorubicin-induced apoptosis. Genet. Mol. Res. 2014, 13, 354–362. [Google Scholar] [CrossRef]
- Xue, H.L.; Huang, X.D.; He, D.; Lin, S.J.; Wang, S.; Niu, T. Effects of Marsdenia tenacissima extract on proliferation and apoptosis of hematologic neoplasm cell line cells. Sichuan Da Xue Xue Bao Yi Xue Ban 2012, 43, 174–179. [Google Scholar]
- Huang, Z.R.; Lin, H.; Wang, Y.; Cao, Z.; Lin, W.; Chen, Q. Studies on the anti-angiogenic effect of Marsdenia tenacissima extract in vitro and in vivo. Oncol. Lett. 2013, 5, 917–922. [Google Scholar]
- Mao, S.L.; Lao, A.N.; Uzawa, J.; Yoshida, S.; Fujimoto, Y. Five new pregnane glycosides from the stems of Marsdenia tenacissima. J. Asian Nat. Prod. Res. 2011, 13, 477–485. [Google Scholar] [CrossRef]
- Luo, S.Q.; Lin, L.Z.; Geoffrey, A.; Xue, L.; Johnson, M.E. Polyoxypregnanes from Marsdenia tenacissima. Phytochemistry 1993, 34, 1615–1620. [Google Scholar] [CrossRef]
- Hu, Y.J.; Shen, X.L.; Lu, H.L.; Zhang, Y.H.; Huang, X.A.; Fu, L.C.; Fong, W.F. Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance in HepG2/Dox cells. J. Nat. Prod. 2008, 71, 1049–1051. [Google Scholar] [CrossRef]
- Ye, B.; Yang, J.; Li, J.; Niu, T.; Wang, S. In vitro and in vivo antitumor activities of tenacissoside C from Marsdenia tenacissima. Planta Med. 2014, 80, 29–38. [Google Scholar]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; García-Regalado, A.; Ruiz, G.; Núñez-Martínez, J.M.; González-Sánchez, I.; Quintanar-Jurado, V.; Morales-Sánchez, E.; Dominguez, F.; López-Toledo, G.; et al. Kaempferitrin induces apoptosis via intrinsic pathway in HeLa cells and exerts antitumor effects. J. Ethnopharmacol. 2013, 145, 476–489. [Google Scholar] [CrossRef]
- Sharan, R.N.; Mehrotra, M.; Choudhury, Y.; Asotra, K. Association of betel nut with carcinogenesis: Revisit with a clinical perspective. PLoS One 2012, 7, e42759. [Google Scholar]
- Von Euw, J.; Reichstein, T. Die Glycoside der Samen von Strophathus Nicholsonii Holm. Glycoside und Aglykone. Helv. Chim. Acta 1948, 31, 888–892. [Google Scholar]
- Hayashi, K.; Wada, K.; Mitsuhashi, H.; Bando, H.; Takase, M.; Terada, S.; Koide, Y.; Aiba, T.; Narita, T.; Mizuno, D. Antitumor active glycosides from Condurango Cortex. Chem. Pharm. Bull. 1980, 28, 1954–1958. [Google Scholar]
- Jiang, Y.; Ahn, E.Y.; Ryu, S.H.; Kim, D.K.; Park, J.S.; Yoon, H.J.; You, S.; Lee, B.J.; Lee, D.S.; Jung, J.H. Cytotoxicity of psammaplin A from a two-sponge association may correlate with the inhibition of DNA replication. BMC Cancer 2004, 4, 70. [Google Scholar] [CrossRef]
- Shen, X.L.; Chen, G.Y.; Zhu, G.Y.; Cai, J.Z.; Wang, L.; Hu, Y.J.; Fong, W.F. 3'-O,4'-O-aromatic acyl substituted 7,8-pyranocoumarins: A new class of P-glycoprotein modulators. J. Pharm. Pharmacol. 2012, 64, 90–100. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, K.; Shen, X.; Jin, W.; Jiang, L.; Saeed Sheikh, M.; Hu, Y.; Huang, Y. Lappaol F, a novel anticancer agent isolated from plant arctium Lappa L. Mol. Cancer Ther. 2014, 13, 49–59. [Google Scholar]
- Li, Q.F.; Wang, X.L.; Ding, L.S.; Zhang, C. Polyoxypregnanes from the stems of Marsdenia tenacissima. Chin. Chem. Lett. 2007, 18, 831–834. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, S.Q. Studies on chemical structures of three new C21-steroidal glycosides from Marsdenia tenacissima. Chin. J. Pharm. 1996, 27, 391–395. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, R.-J.; Shen, X.-L.; Dai, L.-L.; Ai, X.-Y.; Tian, R.-H.; Tang, R.; Hu, Y.-J. Total Aglycones from Marsdenia tenacissima Increases Antitumor Efficacy of Paclitaxel in Nude Mice. Molecules 2014, 19, 13965-13975. https://doi.org/10.3390/molecules190913965
Zhu R-J, Shen X-L, Dai L-L, Ai X-Y, Tian R-H, Tang R, Hu Y-J. Total Aglycones from Marsdenia tenacissima Increases Antitumor Efficacy of Paclitaxel in Nude Mice. Molecules. 2014; 19(9):13965-13975. https://doi.org/10.3390/molecules190913965
Chicago/Turabian StyleZhu, Rui-Jing, Xiao-Ling Shen, Ling-Lin Dai, Xiang-Ying Ai, Ru-Hua Tian, Rong Tang, and Ying-Jie Hu. 2014. "Total Aglycones from Marsdenia tenacissima Increases Antitumor Efficacy of Paclitaxel in Nude Mice" Molecules 19, no. 9: 13965-13975. https://doi.org/10.3390/molecules190913965