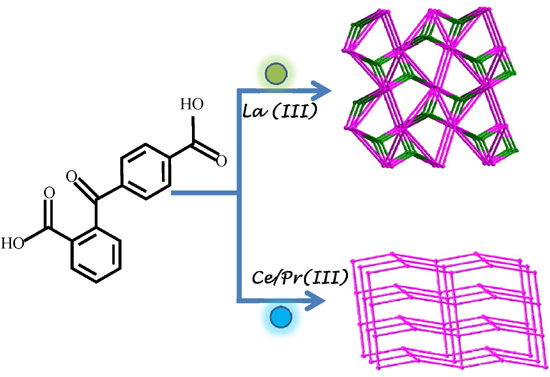
Three Novel Lanthanide Metal-Organic Frameworks (Ln-MOFs) Constructed by Unsymmetrical Aromatic Dicarboxylatic Tectonics: Synthesis, Crystal Structures and Luminescent Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Descriptions
2.1.1. Structure of [La2 (2,4-bpda)3 (H2O)2]n (1)



2.1.2. Structure of {[Ce2 (2,4-bpda)3 (H2O)4]·H2O}n (2)


2.2. Luminescent Properties

2.3. Thermogravimetric Analysis

3. Experimental
3.1. Materials and Physical Measurements
3.2. Synthesis of Complexes 1–3
3.3. X-ray Crystallography
| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical formula | C45H28O17La2 | C45H34O20Ce2 | C45 H32O20 Pr2 |
| Formula weight | 1118.49 | 1174.96 | 1174.53 |
| Temperature | 293 (2) K | 293 (2) K | 293 (2) K |
| Wavelength | 0.71073 | 0.71073 | 0.71073 |
| Crystal system space group | Monoclinic P21/c | Monoclinic Cc | Monoclinic Cc |
| a (Å) | 14.800 (3) | 13.5432 (4) | 13.533 (3) |
| b (Å) | 14.500 (3) | 12.9981 (4) | 12.990 (3) |
| c (Å) | 18.800 (4) | 25.7567 (11) | 25.768 (5) |
| α (°) | 90 | 90 | 90 |
| β (°) | 91.00 (3) | 104.028 (4) | 104.22 (3) |
| γ (°) | 90 | 90 | 90 |
| Volume (Å3) | 4033.9 (14) | 4398.9 (3) | 4391.3 (15) |
| Z Calculated density (Mg·m−3) | 4, 1.842 | 4,1.774 | 4,1.777 |
| Absorption coef. (mm−1) | 2.170 | 2.127 | 2.276 |
| F (000) | 2192 | 2320 | 2320 |
| Crystal size (mm) | 0.20 × 0.17 × 0.14 | 0.32 × 0.27 × 0.23 | 0.50 × 0.35 × 0.30 |
| θ range for data collection (°) | 3.01–25.43 | 3.00–25.50 | 3.11–25.05 |
| Limiting indices | −17 ≤ h ≤ 17 −17 ≤ k ≤ 17 −22 ≤ l ≤ 19 | −16 ≤ h ≤ 16 −15 ≤ k ≤ 15 −21 ≤ l ≤ 31 | −13 ≤ h ≤ 16 −14 ≤ k ≤ 15 −30 ≤ l ≤ 30 |
| reflections collected/unique | 37806/7406 [R (int) = 0.0341] | 9876/6116 [R (int) = 0.0327] | 19982/7245 [R (int) = 0.0306] |
| Max. and min.transmission | 0.7509 and 0.6707 | 0.6405 and 0.5493 | 0.505 and 0.400 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| data/restraints/parameters | 7406/0/577 | 6116/11/605 | 7245/11/617 |
| Goodness-of-fit on F2 | 1.079 | 1.196 | 1.010 |
| R1a, wR2b [I > 2sigma (I)] | 0.0280, 0.0616 | 0.0552, 0.1341 | 0.0245, 0.0578 |
| R indices (all data) | 0.0316, 0.0635 | 0.0568, 0.1350 | 0.0255, 0.0589 |
| Largest diff. peak and hole e.Å−3 | 0.963, −0.658 | 1.971, −2.656 | 0.837, −0.539 |
| 1 | 2 | 3 | |||
|---|---|---|---|---|---|
| La1-O8B | 2.404 (2) | Ce1-O14C | 2.417 (13) | Pr1-O1 | 2.454 (3) |
| La1-O13D | 2.480 (2) | Ce1-O7 | 2.457 (12) | Pr1-O3A | 2.441 (4) |
| La1-O7 | 2.487 (2) | Ce1-O16 | 2.504 (11) | Pr1-O6 | 2.417 (3) |
| La1-O3A | 2.498 (2) | Ce1-O1 | 2.572 (12) | Pr1-O9B | 2.408 (3) |
| La1-O1 | 2.582 (2) | Ce2-O8B | 2.416 (10) | Pr1-O14C | 2.479 (4) |
| La1-O12 | 2.577 (3) | Ce2-O11 | 2.506 (11) | Pr1-O13C | 2.551 (4) |
| La1-O16 | 2.630 (3) | Ce2-O4A | 2.526 (12) | Pr1-O16 | 2.505 (3) |
| La1-O2 | 2.689 (2) | Ce2-O19 | 2.561 (11) | Pr1-O17 | 2.557 (4) |
| La1-O11 | 2.688 (2) | Ce2-O12 | 2.866 (10) | Pr2-O2 | 2.429 (3) |
| La2-O9C | 2.402 (3) | O4-Ce2 | 2.526 (12) | Pr2-O4A | 2.414 (3) |
| La2-O4A | 2.410 (2) | O9-Ce1C | 2.501 (12) | Pr2-O6 | 2.822 (4) |
| La2-O14E | 2.434 (2) | O14-Ce1B | 2.417 (13) | Pr2-O7 | 2.478 (3) |
| La2-O6 | 2.454 (2) | Ce1-O12 | 2.427 (10) | Pr2-O8B | 2.514 (4) |
| La2-O17 | 2.560 (3) | Ce1-O9B | 2.501 (12) | Pr2-O11 | 2.529 (3) |
| La2-O2 | 2.560 (2) | Ce1-O2 | 2.516 (12) | Pr2-O12 | 2.529 (3) |
| La2-O15 | 2.586 (2) | Ce1-O17 | 2.595 (13) | Pr2-O18 | 2.548 (3) |
| La2-O11 | 2.605 (2) | Ce2-O6 | 2.455 (12) | Pr2-O19 | 2.566 (4) |
| O3-La1A | 2.498 (2) | Ce2-O13C | 2.506 (13) | O3-Pr1B | 2.441 (4) |
| O4-La2A | 2.410 (2) | Ce2-O3A | 2.531 (12) | O4-Pr2B | 2.414 (3) |
| O8-La1B | 2.404 (2) | Ce2-O18 | 2.592 (12) | O8-Pr2A | 2.514 (4) |
| O9-La2 | 2.401 (3) | O3-Ce2 | 2.531 (12) | O9-Pr1A | 2.408 (3) |
| O13-La1D | 2.480 (2) | O8-Ce2C | 2.416 (10) | O13-Pr1 | 2.551 (4) |
| O14-La2 | 2.434 (2) | O13-Ce2B | 2.506 (13) | O14-Pr1 | 2.479 (4) |
| O8B-La1-O13D | 74.76 (8) | O14C-Ce1-O12 | 73.3 (4) | O9B-Pr1-O6 | 72.48 (12) |
| O8B-La1-O7 | 146.86 (8) | O12-Ce1-O7 | 122.2 (3) | O9B-Pr1-O3A | 129.47 (11) |
| O13D-La1-O7 | 78.79 (8) | O12-Ce1-O9B | 79.9 (4) | O6-Pr1-O3A | 79.60 (12) |
| O8B-La1-O3A | 81.85 (8) | O14C-Ce1-O16 | 141.7 (4) | O9B-Pr1-O1 | 79.78 (13) |
| O13D-La1-O3A | 134.34 (9) | O7-Ce1-O16 | 74.4 (4) | O6-Pr1-O1 | 120.74 (11) |
| O7-La1-O3A | 131.28 (8) | O14C-Ce1-O2 | 86.7 (4) | O3A-Pr1-O1 | 79.62 (12) |
| O8B-La1-O1 | 79.13 (8) | O7-Ce1-O2 | 127.0 (4) | O9B-Pr1-O14C | 85.92 (14) |
| O13D-La1-O1 | 136.64 (9) | O16-Ce1-O2 | 86.8 (4) | O6-Pr1-O14C | 101.33 (12) |
| O7-La1-O1 | 108.27 (8) | O12-Ce1-O1 | 143.8 (4) | O3A-Pr1-O14C | 141.37 (13) |
| O3A-La1-O1 | 73.37 (8) | O9B-Ce1-O1 | 136.3 (4) | O1-Pr1-O14C | 127.98 (12) |
| O8B-La1-O12 | 96.35 (8) | O2-Ce1-O1 | 51.6 (4) | O9B-Pr1-O16 | 142.41 (12) |
| O13D-La1-O12 | 70.19 (9) | O12-Ce1-O17 | 76.7 (4) | O6-Pr1-O16 | 144.93 (12) |
| O7-La1-O12 | 93.10 (9) | O9B-Ce1-O17 | 74.7 (4) | O3A-Pr1-O16 | 72.64 (12) |
| O3A-La1-O12 | 74.20 (9) | O2-Ce1-O17 | 67.7 (4) | O1-Pr1-O16 | 75.11 (13) |
| O1-La1-O12 | 147.57 (9) | O8B-Ce2-O6 | 72.7 (4) | O14C-Pr1-O16 | 87.92 (14) |
| O8B-La1-O16 | 81.94 (9) | O6-Ce2-O11 | 124.3 (4) | O9B-Pr1-O13C | 80.67 (13) |
| O13D-La1-O16 | 75.75 (10) | O6-Ce2-O13C | 76.3 (4) | O6-Pr1-O13C | 143.48 (13) |
| O7-La1-O16 | 72.39 (9) | O8B-Ce2-O4A | 124.4 (4) | O3A-Pr1-O13C | 136.89 (12) |
| O3A-La1-O16 | 139.01 (9) | O11-Ce2-O4A | 144.2 (4) | O1-Pr1-O13C | 76.74 (12) |
| O1-La1-O16 | 66.71 (9) | O8B-Ce2-O3A | 73.5 (4) | O14C-Pr1-O13C | 51.56 (12) |
| O12-La1-O16 | 145.04 (10) | O11-Ce2-O3A | 129.3 (4) | O16-Pr1-O13C | 66.72 (13) |
| O8B-La1-O2 | 126.76 (8) | O4A-Ce2-O3A | 51.5 (4) | O9B-Pr1-O17 | 134.60 (12) |
| O13D-La1-O2 | 151.47 (7) | O6-Ce2-O19 | 141.6 (4) | O6-Pr1-O17 | 77.37 (13) |
| O7-La1-O2 | 73.89 (7) | O13C-Ce2-O19 | 67.6 (4) | O3A-Pr1-O17 | 75.22 (12) |
| O3A-La1-O2 | 72.31 (8) | O3A-Ce2-O19 | 116.3 (4) | O1-Pr1-O17 | 145.56 (13) |
| O1-La1-O2 | 49.39 (7) | O6-Ce2-O18 | 146.4 (4) | O14C-Pr1-O17 | 67.54 (13) |
| O12-La1-O2 | 118.90 (7) | O13C-Ce2-O18 | 136.9 (4) | O16-Pr1-O17 | 75.18 (13) |
| O16-La1-O2 | 88.15 (9) | O3A-Ce2-O18 | 67.8 (4) | O13C-Pr1-O17 | 106.65 (13) |
| O8B-La1-O11 | 139.67 (8) | O8B-Ce2-O12 | 70.8 (4) | O4A-Pr2-O2 | 73.30 (12) |
| O13D-La1-O11 | 104.25 (9) | O11-Ce2-O12 | 48.3 (3) | O4A-Pr2-O7 | 76.62 (13) |
| O7-La1-O11 | 66.45 (8) | O4A-Ce2-O12 | 158.3 (3) | O2-Pr2-O7 | 124.40 (11) |
| O3A-La1-O11 | 70.41 (8) | O19-Ce2-O12 | 97.9 (3) | O4A-Pr2-O8B | 131.44 (11) |
| O1-La1-O11 | 117.95 (8) | O14C-Ce1-O7 | 79.7 (4) | O2-Pr2-O8B | 76.60 (12) |
| O12-La1-O11 | 48.81 (7) | O14C-Ce1-O9B | 129.8 (4) | O7-Pr2-O8B | 90.55 (13) |
| O16-La1-O11 | 137.79 (9) | O7-Ce1-O9B | 80.0 (4) | O4A-Pr2-O12 | 124.23 (12) |
| O2-La1-O11 | 72.19 (7) | O12-Ce1-O16 | 144.9 (3) | O2-Pr2-O12 | 91.50 (12) |
| O9C-La2-O4A | 80.18 (10) | O9B-Ce1-O16 | 72.7 (4) | O7-Pr2-O12 | 143.72 (12) |
| O9C-La2-O14E | 76.44 (9) | O12-Ce1-O2 | 101.5 (4) | O8B-Pr2-O12 | 93.42 (11) |
| O4A-La2-O14E | 87.56 (9) | O9B-Ce1-O2 | 140.8 (4) | O4A-Pr2-O11 | 73.29 (11) |
| O9C-La2-O6 | 121.04 (9) | O14C-Ce1-O1 | 80.5 (4) | O2-Pr2-O11 | 82.69 (12) |
| O4A-La2-O6 | 132.95 (8) | O7-Ce1-O1 | 75.6 (4) | O7-Pr2-O11 | 130.43 (13) |
| O14E-La2-O6 | 135.64 (8) | O16-Ce1-O1 | 66.1 (4) | O8B-Pr2-O11 | 138.60 (12) |
| O9C-La2-O17 | 82.72 (10) | O14C-Ce1-O17 | 135.3 (4) | O12-Pr2-O11 | 51.38 (11) |
| O4A-La2-O17 | 158.67 (9) | O7-Ce1-O17 | 145.0 (4) | O4A-Pr2-O18 | 142.50 (12) |
| O14E-La2-O17 | 76.06 (9) | O16-Ce1-O17 | 75.2 (4) | O2-Pr2-O18 | 141.79 (12) |
| O6-La2-O17 | 67.47 (8) | O1-Ce1-O17 | 107.2 (4) | O7-Pr2-O18 | 70.90 (12) |
| O9C-La2-O2 | 80.49 (8) | O8B-Ce2-O11 | 75.8 (4) | O8B-Pr2-O18 | 67.90 (11) |
| O4A-La2-O2 | 76.23 (9) | O8B-Ce2-O13C | 130.4 (4) | O12-Pr2-O18 | 77.33 (12) |
| O14E-La2-O2 | 153.75 (8) | O11-Ce2-O13C | 91.6 (5) | O11-Pr2-O18 | 115.16 (11) |
| O6-La2-O2 | 68.07 (7) | O6-Ce2-O4A | 91.3 (4) | O4A-Pr2-O19 | 81.80 (13) |
| O17-La2-O2 | 113.38 (9) | O13C-Ce2-O4A | 93.6 (4) | O2-Pr2-O19 | 145.77 (13) |
| O9C-La2-O15 | 145.33 (8) | O6-Ce2-O3A | 82.8 (4) | O7-Pr2-O19 | 69.81 (12) |
| O4A-La2-O15 | 108.74 (10) | O13C-Ce2-O3A | 138.8 (4) | O8B-Pr2-O19 | 137.33 (13) |
| O14E-La2-O15 | 70.72 (8) | O8B-Ce2-O19 | 142.6 (4) | O12-Pr2-O19 | 83.32 (12) |
| O6-La2-O15 | 78.08 (8) | O11-Ce2-O19 | 70.8 (4) | O11-Pr2-O19 | 67.66 (12) |
| O17-La2-O15 | 78.93 (10) | O4A-Ce2-O19 | 78.6 (4) | O18-Pr2-O19 | 69.90 (13) |
| O2-La2-O15 | 133.91 (7) | O8B-Ce2-O18 | 83.4 (5) | O4A-Pr2-O6 | 71.46 (11) |
| O9C-La2-O11 | 146.07 (8) | O11-Ce2-O18 | 69.4 (4) | O2-Pr2-O6 | 78.07 (11) |
| O4A-La2-O11 | 70.99 (9) | O4A-Ce2-O18 | 83.0 (4) | O7-Pr2-O6 | 48.17 (10) |
| O14E-La2-O11 | 118.66 (8) | O19-Ce2-O18 | 69.6 (4) | O8B-Pr2-O6 | 65.60 (11) |
| O6-La2-O11 | 71.23 (8) | O6-Ce2-O12 | 78.1 (3) | O12-Pr2-O6 | 158.11 (11) |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rocha, J.; Carlos, L.D.; Paz, F.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Gai, Y.L.; Xiong, K.C.; Chen, L.; Bu, Y.; Li, X.J.; Jiang, F.L.; Hong, M.C. Visible and NIR photoluminescence properties of a series of novel lanthanide-organic coordination polymers based on hydroxyquinoline-carboxylate ligands. Inorg. Chem. 2012, 51, 13128–13137. [Google Scholar] [CrossRef]
- Pan, M.; Zheng, X.L.; Liu, Y.; Liu, W.S.; Su, C.Y. Structural and photoluminescent studies of lanthanide complexes with tripodal triRNTB (N-substituted tris (benzimidazol-2-ylmethyl)amine): Ligand substituent, anionic and secondary ligand effects. Dalton Trans. 2009, 2157–2169. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jiang, Y.L.; Xiahou, Z.J.; Fu, J.H.; Liu, Q.Y. Diversity of lanthanide (III)-2,5-dihydroxy-1,4-benzenedicarboxylate extended frameworks: Syntheses, structures, and magnetic properties. Dalton Trans. 2012, 11428–11437. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Zhao, J.; Long, L.S.; Huang, R.B.; Zheng, L.S. A lanthanide-based metal-organic framework with a dynamic porous property. Dalton Trans. 2008, 4714–4716. [Google Scholar] [CrossRef]
- Fu, F.; Li, D.S.; Wu, Y.P.; Gao, X.M.; Du, M.; Tang, L.; Zhang, X.N.; Meng, C.X. A versatile V-shaped tetracarboxylate building block for constructing mixed-ligand Co (II) and Mn (II) complexes incorporating various N-donor co-ligands. CrystEngComm 2010, 12, 1227–1237. [Google Scholar] [CrossRef]
- Li, D.S.; Wu, Y.P.; Zhao, J.; Zhang, J.; Lu, J.Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014, 261, 1–27. [Google Scholar] [CrossRef]
- Hu, S.; Yun, L.; Zheng, Y.Z.; Lan, Y.H.; Powell, A.K.; Tong, M.L. Ferrimagnetic [CoII3 (μ3-OH)2 (RCO2)4] chains embedded in a laminar hybrid material exhibiting single-chain magnet behaviour. Dalton Trans. 2009, 1897–1900. [Google Scholar] [CrossRef]
- Yang, X.G.; Li, D.S.; Fu, F.; Wu, Y.P.; Wang, J.J.; Wang, Y.Y. 1D Ladder-like Chain and 1D Channeled 3D Supramolecular Architectures Based on Benzophenone-2,4ꞌ-dicarboxylic Acid. Chin. J. Chem. 2008, 26, 655–660. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.L.; Meng, Z.S.; Zheng, Y.Z.; Lan, Y.H.; Powell, A.K.; Tong, M.L. Pentacobalt (II) cluster based pcu network exhibits both magnetic slow-relaxation and hysteresis behaviour. Dalton Trans. 2011, 27–30. [Google Scholar]
- Xu, J.K.; Sun, X.C.; Fan, Y.H.; Bi, C.F.; Sun, M. Hydrothermal synthesis of five new coordination polymers based on benzophenone-2,4-dicarboxylic Acid and N-donor spacers. Z. Anorg. Allg. Chem. 2012, 638, 1512–1518. [Google Scholar] [CrossRef]
- Chen, P.X.; Yang, G.P.; Hou, L.; Wang, Y.Y.; Shi, Q.Z. Synthesis, structures, and properties of lead (II) and cobalt (II) metal-organic frameworks based on a flexible benzophenone-2,4'-dicarboxylic acid (H2bpdc). J. Coord. Chem. 2012, 65, 2893–2902. [Google Scholar] [CrossRef]
- Wang, J.; Tao, J.Q.; Xu, X.J.; Mao, D. A CdSO4-like 3D framework constructed from benzophenone-2,4'-dicarboxylic acid and 1,4-bis (1,2,4-triazol-1-ylmethyl)-benzene: Synthesis, structure and physical properties. Bull. Korean Chem. Soc. 2013, 34, 2191–2194. [Google Scholar] [CrossRef]
- Weng, S.F.; Wang, Y.H.; Lee, C.S. New metal-organicframeworksof [M∙(C6H5O7)∙(C6H6O7)∙(C6H7O7)∙(H2O)]∙H2O (M = La, Ce) and [Ce2∙(C2O4)∙(C6H6O7)2]·4H2O. J. Solid State Chem. 2012, 188, 77–83. [Google Scholar] [CrossRef]
- Jia, L.N.; Hou, L.; Wei, L.; Jing, X.J.; Liu, B.; Wang, Y.Y.; Shi, Q.Z. Five sra Topological Ln (III)-MOFs Based on Novel Metal-Carboxylate/Cl Chain: Structure, Near-Infrared Luminescence and Magnetic Properties. Cryst. Growth Des. 2013, 13, 1570–1576. [Google Scholar]
- Li, D.S.; Zhang, P.; Zhao, J.; Fang, Z.F.; Du, M.; Zou, K.; Mu, Y.Q. Two unique entangling CdII-coordination frameworks constructed by square Cd4-building blocks and auxiliary N,N'-donor ligands. Cryst. Growth Des. 2012, 12, 1697–1702. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Feng, X.; Wen, Y.H.; Lan, Y.Z.; Feng, Y.L.; Pan, C.Y.; Yao, Y.G. Multifunctional zinc (II) urocanate with rare fivefold interpenetrating diamondoid network. Inorg. Chem. Commun. 2009, 12, 89–91. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS 97,Program for Crystal Structure Solution and Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SADABS, a Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds {[Ln2 (2,4-bpda)3 (H2O)x]·yH2O}n (Ln = La (III) (1), x = 2, y = 0, Ce (III) (2), Pr (III) (3), x = 4, y = 1) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, Y.-P.; Li, D.-S.; Xia, W.; Guo, S.-S.; Dong, W.-W. Three Novel Lanthanide Metal-Organic Frameworks (Ln-MOFs) Constructed by Unsymmetrical Aromatic Dicarboxylatic Tectonics: Synthesis, Crystal Structures and Luminescent Properties. Molecules 2014, 19, 14352-14365. https://doi.org/10.3390/molecules190914352
Wu Y-P, Li D-S, Xia W, Guo S-S, Dong W-W. Three Novel Lanthanide Metal-Organic Frameworks (Ln-MOFs) Constructed by Unsymmetrical Aromatic Dicarboxylatic Tectonics: Synthesis, Crystal Structures and Luminescent Properties. Molecules. 2014; 19(9):14352-14365. https://doi.org/10.3390/molecules190914352
Chicago/Turabian StyleWu, Ya-Pan, Dong-Sheng Li, Wei Xia, Sha-Sha Guo, and Wen-Wen Dong. 2014. "Three Novel Lanthanide Metal-Organic Frameworks (Ln-MOFs) Constructed by Unsymmetrical Aromatic Dicarboxylatic Tectonics: Synthesis, Crystal Structures and Luminescent Properties" Molecules 19, no. 9: 14352-14365. https://doi.org/10.3390/molecules190914352