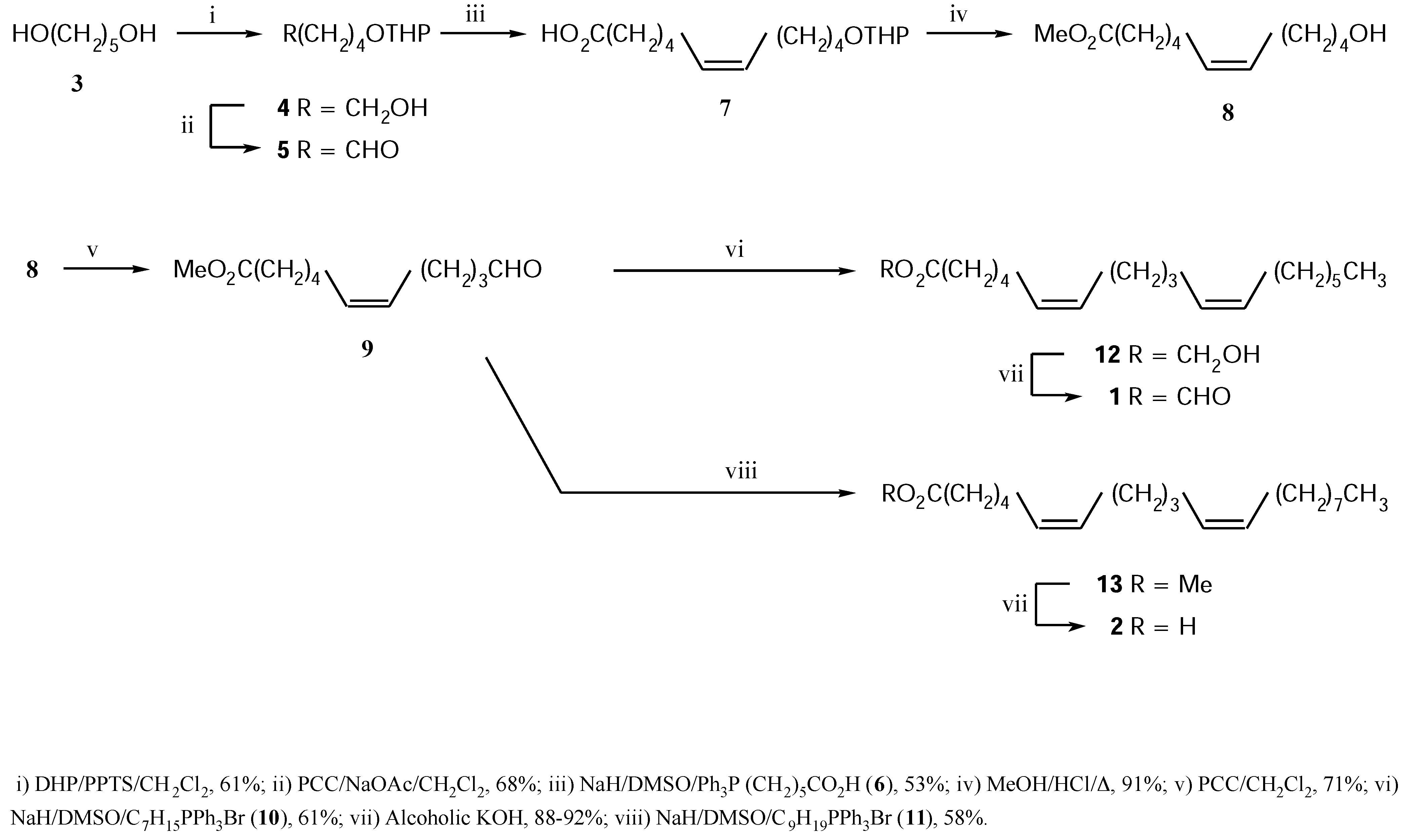Results and Discussion
The synthesis was based on a “building-block” approach consisting of coupling between C
5- and C
6-units to furnish the common intermediate
9. Subsequent addition of the appropriate C
7- and C
9-moieties to it gives
1 and
2 respectively after proper functionalization. The stereo-selectivities of the incipient olefins were fixed by
Z-selective Wittig reactions (
Scheme 1).
Commercially available, pentane-1,5-diol (
3) was monopyranylated to the compound
4 which on oxidation with “buffered PCC” [
9] gave the aldehyde
5. Its
Z-selective Wittig olefination [
10] with the known phosphor-ane generated from
6 [
11] furnished compound
7. Although Wittig reactions with carboxylic acids bearing phosphoranes are reported in the literature [
12], we encountered difficulty in the isolation step leading to poor yield of the Wittig product. Consequently, a modified work-up was employed (see
Experimental). Acidic deprotection of
7 led to the hydroxy compound
8 with concomitant esterification. Its oxidation followed by a second Wittig reaction of the resultant alde-hyde
9 with the C
7-phosphorane, generated from
10 [
13] under the above condition afforded the ester
12 with 97% isomeric purity (by capillary GLC analysis). This was converted to
1 by alkaline hydrolysis.
The (
Z)-geometry of the two olefinic bonds was established by the absence of any IR band at 960-980 cm
−1. Further confirmation of this was accomplished by the
13C NMR spectrum of
12 which exhibited signals due to the allylic carbons at δ 27.2 and 27.8 ppm, characteristic of the internal (
Z)-alkenes [
14,
15].
Likewise, the Wittig reaction of
9 with C
9-phos-phorane, generated from
11 [
16] gave
13 with 98% isomeric purity (by capillary GLC analysis) whose exclusive (
Z)-geometry was also confirmed by
13C NMR analysis as above. Its alkaline hydrolysis afforded the acid
2. The mass spectral data of
1 and
2 were consistent with the reported values [
3].
Likewise, the Wittig reaction of
9 with C
9-phos-phorane, generated from
11 [
16] gave
13 with 98% isomeric purity (by capillary GLC analysis) whose exclusive (
Z)-geometry was also confirmed by
13C NMR analysis as above. Its alkaline hydrolysis afforded the acid
2. The mass spectral data of
1 and
2 were consistent with the reported values [
3].
Experimental Section
All bps are uncorrected. The IR spectra were scanned with a Perkin-Elmer 783 spectrophotometer. The PMR spectra were recorded in CDCl3 with a Bruker AC-200 (200 MHz) spectrometer. The mass spectra (70 eV) were recorded with a Shimadzu GCMS-QP 1000A spectrometer using the direct probe injection. The GLC analyses were carried out on a Shimadzu GC-16A chromatograph fitted with a flame ionization detector and a quartz capillary column (OV-17). Anhydrous reactions were carried out under Ar using freshly dried solvents. All organic extracts were dried over anhydrous Na2SO4.
5-(2-Tetrahydropyranyloxy)-pentan-1-ol (4)
A mixture of 3 (10.0 g, 0.096 mol), PPTS (0.2 g) and dihydropyran (8.1 g, 0.096 mol) in CH2Cl2 (50 mL) was stirred at 0 °C for 2 h and at room temperature for an additional 2 h. It was then poured into aqueous NaHCO3 and extracted with ether. Usual isolation followed by column chromatography over neutral alumina (gr. II) eluting it with 0-20% EtOAc/hexane afforded pure 5 (11.0 g, 61%) along with a little dipyranylated product: bp 92-94 °C/0.2 mm; IR: 3400, 1010, 900, 860, 800 cm−1; PMR: δ 1.5 (br. s, 12H), 2.23 (s, D2O exchangeable OH, 1H), 3.3-4.2 (m, 6H), 4.63 (s, 1H); Anal. Calcd. C10H20O3: C, 63.79; H, 10.71; Found: C, 63.57; H, 10.89.
5-(2-Tetrahydropyranyloxy)-pentanal (5)
Oxidation of 4 (8.0 g, 0.043 mol) with PCC (13.9 g, 0.065 mol) in presence of NaOAc (0.41 g, 5.0 mmol) in CH2Cl2 (40 mL) furnished the aldehyde 5 (5.4 g, 68%) which was found to be reasonably pure and used as such for the next step due to its instability: IR: 2720, 1730, 1005, 910, 860, 805 cm−1; PMR: δ 1.4 (br. s, 10H), 2.3-2.6 (m, 2H), 3.3-4.1 (m, 4H), 4.60 (s, 1H), 9.8 (t, J = 1.5 Hz, 1H).
(6Z)-11-(2-Tetrahydropyranyloxy)-undec-6-enoic acid (7)
To a stirred solution of 6 (16.9 g, 0.037 mol) in DMSO (20 mL) at room temperature was added a solution of dimsyl solution (0.074 mol, prepared separately by heating NaH and DMSO to 55 °C) in DMSO (30 mL) at room temperature. After 1 h, the aldehyde 5 (5.2 g, 0.028 mol) in DMSO (10 mL) was added to the resulting red solution and stirring continued for 18 h at the same temperature. Most of the solvent was removed at 35-40 °C under 0.1 mm vacuum, water added to the residue and the content extracted with EtOAc. The aqueous extract was acidified with 50% aqueous oxalic acid to pH 2 and reextracted with ether-hexane (1:1). The extract was washed with water and brine and dried. Removal of solvent followed by column chromatography of the residue (silica gel, 0-30% EtOAc/hexane) afforded compound 7 (4.2 g, 53%): IR: 3700-3500, 1710, 1010, 910, 860, 800 cm−1; PMR: δ 1.2-1.7 (m, 14H), 1.9-2.1 (m, 4H), 2.3 (t, J = 6 Hz, 2H), 3.2-3.6 (m, 4H), 4.65 (s, 1H), 5.3-5.5 (m, 2H), 9.5 (br. s, D2O exchangeable, 1H); Anal. Calcd. C16H28O4: C, 67.57; H, 9.92; Found: C, 67.68; H, 9.89.
Methyl (6Z)-11-hydroxyundec-6-enoate (8)
A solution of 7 (4.1 g, 0.014 mol) in MeOH (100 mL) containing HCl (2N, 3-4 drops) was refluxed for 8 h. Most of the solvent was removed in vacuo, the residue was taken up in ether and the organic extract washed with water and brine and dried. Removal of solvent gave pure 8 (2.8 g, 91%): IR: 3400, 1740, 1655 cm−1; PMR: δ 1.4-1.7 (m, 8H), 1.83 (br. s, D2O exchangeable, 1H), 1.9-2.5 (m, 6H), 3.60 (s, 3H), 3.72 (t, J = 6 Hz, 2H), 5.4-5.6 (m, 2H). Anal. Calcd. C12H22O3: C, 67.25; H, 10.35; Found: C, 67.08; H, 10.22.
(5Z)-10-Carbomethoxydec-5-enal (9)
As described earlier, compound 8 (2.8 g, 0.013 mol) was oxidized with PCC (4.3 g, 0.02 mol) in CH2Cl2 (30 mL) to give the aldehyde 9 (1.96 g, 71%): IR: 2720, 1740, 1715, 1660 cm−1; PMR: δ 1.2-1.8 (m, 6H), 1.9-2.2 (m, 4H), 2.3-2.5 (m, 4H), 3.66 (s, 3H), 5.3-5.5 (m, 2H), 9.7 (t, J = 1.5 Hz, 1H).
(6Z,11Z)-Octadeca-6,11-dienoic acid (1)
Wittig olefination between 9 (0.98 g, 4.6 mmol) and the phosphonium salt 10 (2.65 g, 6.0 mmol) using dimsyl ion as the base gave the ester 12 (0.82 g, 61%): glc (quartz capillary column OV-17, 50 Mt., id. 0.25 mm, split 1:100, FID, N2 2 mL/min, temp. 210 °C): tR = 13.20 min (97%); IR: 1740, 1640 cm−1; PMR: δ 0.87 (dist. t, 3H), 1.2-1.4 (m, 14H), 1.9-2.1 (m, 8H), 2.31 (t, J = 7.5 Hz, 2H), 3.66 (s, 3H), 5.3-5.5 (m, 4H); 13C NMR: δ 14.0, 22.6, 24.5, 26.8, 27.2, 28.9, 29.1, 29.7, 29.8, 30.7, 31.7, 33.9, 51.4, 129.1, 129.4, 130.1 130.2, 174.1. Anal. Calcd. C19H34O2: C, 77.49; H, 11.64; Found: C, 77.28; H, 11.78.
The above ester 12 (0.5 g, 1.7 mmol) was hydrolyzed with alcoholic KOH (2N). The usual work-up followed by column chromatography (silica gel, 0-30% EtOAc/hexane) of the crude product gave 1 (0.44 g, 92%): IR: 3600-3400, 1720 cm−1; PMR: δ 0.87 (dist. t, 3H), 1.2-1.5 (m, 14H), 1.9-2.2 (m, 8H), 2.32 (t, J = 6 Hz, 2H), 5.3-5.5 (m, 4H), 8.6 (br. s, D2O exchangeable, 1H). Anal. Calcd. C18H32O2: C, 77.09; H, 11.50; Found: C, 76.96; H, 11.42.
(6Z,11Z)-Eicosa-6,11-dienoic acid (2)
As above, reaction between 9 (0.98 g, 4.6 mmol) and the phosphonium salt 11 (2.8 g, 6.0 mmol) gave the ester 13 (0.86 g, 58%): glc (quartz capillary column OV-17, 50 Mt., id. 0.25 mm, split 1:100, FID, N2 2 mL/min, temp. 210 °C): tR = 17.85 min (98%); IR: 1735, 1660 cm−1; PMR: δ 0.88 (dist. t, 3H), 1.2-1.4 (m, 18H), 1.9-2.1 (m, 8H), 2.31 (t, J = 7.5 Hz, 2H), 3.68 (s, 3H), 5.3-5.5 (m, 4H); 13C NMR: δ 14.1, 22.6, 24.5, 25.5, 26.8, 27.2, 27.6, 29.3, 29.5, 29.7, 30.7, 31.6, 34.0, 51.4, 129.2, 129.4, 130.1 130.2, 174.1. Anal. Calcd. C21H38O2: C, 78.20; H, 11.88; Found: C, 78.17; H, 11.97.
Hydrolysis of 13 (0.5 g, 1.6 mmol) with alcoholic KOH (2N) followed by usual work-up and column chromatography (silica gel, 0-30% EtOAc/hexane) of the crude product gave 2 (0.42 g, 88%): IR: 3700-3500, 1715 cm−1; PMR: δ 0.89 (dist. t, 3H), 1.2-1.6 (m, 18H), 1.9-2.2 (m, 8H), 2.35 (t, J = 6 Hz, 2H), 5.4-5.6 (m, 4H), 9.8 (br. s, D2O exchangeable, 1H). Anal. Calcd. C20H36O2: C, 77.86; H, 11.76; Found: C, 77.75; H, 11.83.