Synthesis of New 2-(1,3-Dithianyl)phenols and Hexakis-[p-(1,3-dithian-2-yl)phenoxy]cyclotriphosphazene
Abstract
:Introduction
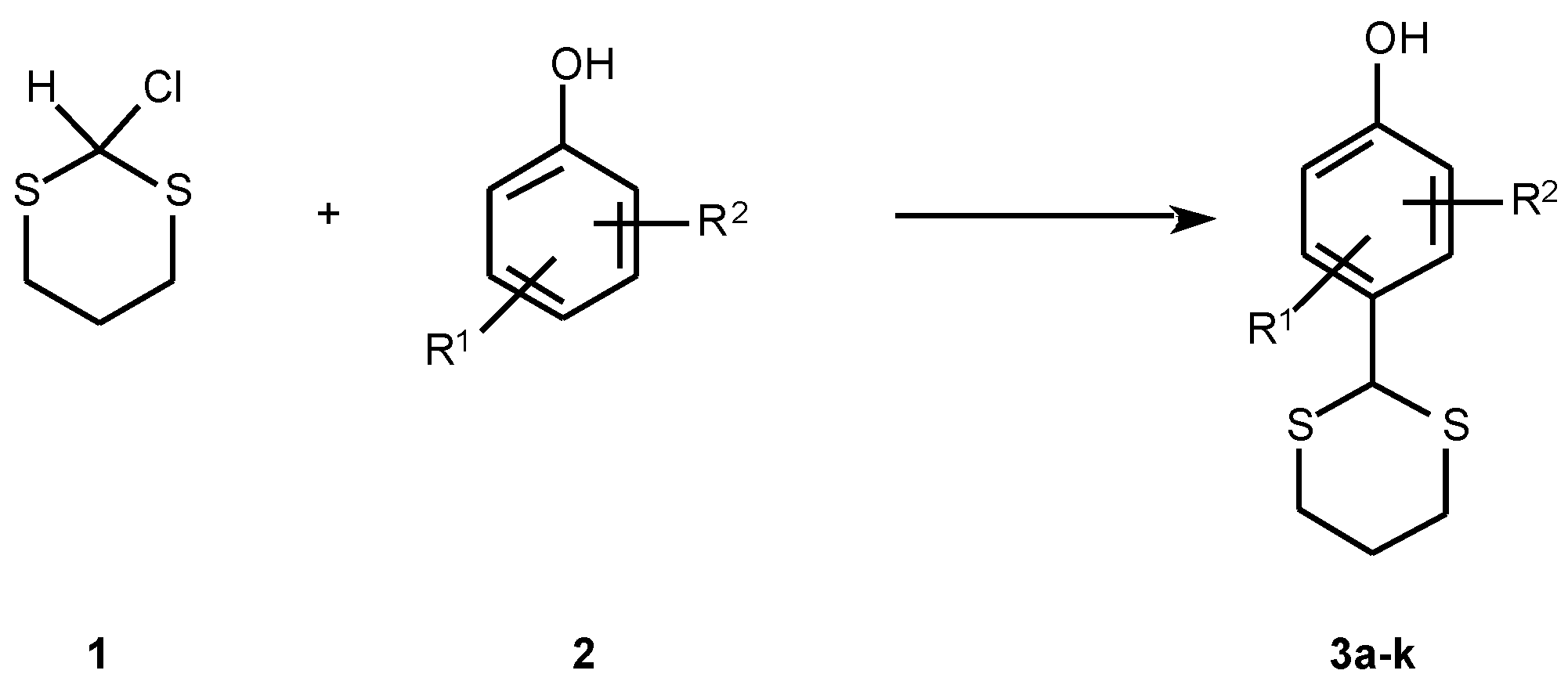
Results and Discussion
| Compound | R1 | R2 | R | Formula Mr | M.p. °C | Yield % | Calcd/found | ||
|---|---|---|---|---|---|---|---|---|---|
| C | H | S | |||||||
| 3a | 2-CH3 | 6-CH3 | 4-dithianyl | C12H16OS2 | 182-185 | 43 | 60.00 | 6.71 | 26.69 |
| 240.24 | 59.78 | 6.56 | 26.20 | ||||||
| 3b | 3-CH3 | 4-CH3 | 6-dithianyl | C12H16OS2 | 129-132 | 23 | 60.00 | 6.71 | 26.69 |
| 240.24 | 59.45 | 6.42 | 26.34 | ||||||
| 3c | 3-CH3 | 5-C2H5 | 4-dithianyl | C13H18OS2 | 139-141 | 24 | 61.42 | 7.14 | 25.22 |
| 254.25 | 61.05 | 6.88 | 25.05 | ||||||
| 3d | 2-sec-C4H9 | H | 4-dithianyl | C14H20OS2 | 96-98 | 19 | 62.68 | 7.51 | 23.90 |
| 268.26 | 62.51 | 7.33 | 23.85 | ||||||
| 3e | 3-tert-C4H9 | H | 6-dithianyl | C14H20OS2 | 112-114 | 43 | 62.68 | 7.51 | 23.90 |
| 268.26 | 62.44 | 7.24 | 23.57 | ||||||
| 3f | 2-C6H12 | 4-dithianyl | C16H22OS2 | 120-123 | 30 | 65.31 | 7.48 | 21.79 | |
| 294.23 | 65.11 | 7.50 | 21.66 | ||||||
| 3g | 2-CH3O | H | 4-dithianyl | C11H14O2S2 | 140-143 | 29 | 54.54 | 5.83 | 26.47 |
| 242.23 | 54.74 | 5.67 | 26.25 | ||||||
| 3h | 3-iso-C3H7O | H | 4-dithianyl | C13H18O2S2 | 119-121 | 19 | 57.78 | 6.71 | 23.73 |
| 270.25 | 57.26 | 6.48 | 23.60 | ||||||
| 3i | 2-Cl | H | 6-dithianyl | C10H11ClOS2 | 103-106 | 17 | 48.69 | 4.49 | 25.99 |
| 246.67 | 48.30 | 4.45 | 25.75 | ||||||
| 3j | 4-Br | H | 2-dithianyl | C10H11BrOS2 | 136-138 | 33 | 41.25 | 3.81 | 22.02 |
| 291.20 | 40.97 | 3.34 | 21.74 | ||||||
| 3k | 3-F | H | 4-dithianyl | C10H11FOS2 | 122-123 | 22 | 51.98 | 4.80 | 27.75 |
| 231.09 | 51.59 | 4.78 | 27.58 | ||||||
Experimental
| Compound | 13C NMR chemical shifts, δ in ppm |
|---|---|
| 3a | 152.3, 130.7, 127.9, 123.3, 51.0, 32.3, 25.2, 15.9 |
| 3b | 151.6, 138.5, 129.6, 128.6, 120.8, 118.1, 46.4, 31.7, 24.8, 19.4 |
| 3c | 154.7, 141.1, 137.3, 115.2, 113.6, 48.4, 33.0, 28.1, 25.6, 21.4, 15.6 |
| 3d | 153.2, 133.6, 131.3, 126.7, 125.9, 115.4, 51.2, 34.0, 32.3, 29.6, 25.0 |
| 3e | 153.7, 153.5, 128.5, 120.5, 117.8, 114.3, 46.7, 34.5, 31.5, 31.1, 24.7 |
| 3f | 152.8, 133.9, 131.3, 126.4, 125.9, 115.4, 51.1, 37.2, 32.8, 32.2, 26.9, 26.2 |
| 3g | 146.5, 145.7, 131.0, 120.8, 114.4, 110.2, 55.9, 51.2, 32.2, 25.0 |
| 3h | 156.2, 155.7, 130.1, 120.4, 115.0, 102.6, 71.1, 47.7, 43.0, 32.4, 26.8 |
| 3i | 151.3, 132.4, 128.4, 128.0, 119.4, 116.3, 50.0, 32.0, 24.9 |
| 3j | 153.6, 132.8, 131.7, 125.8, 119.1, 112.5, 46.6, 31.5, 24.6 |
| 3k | 164.3, 157.0, 130.2, 118.4, 112.0, 102.7, 42.7, 32.2, 24.9 |
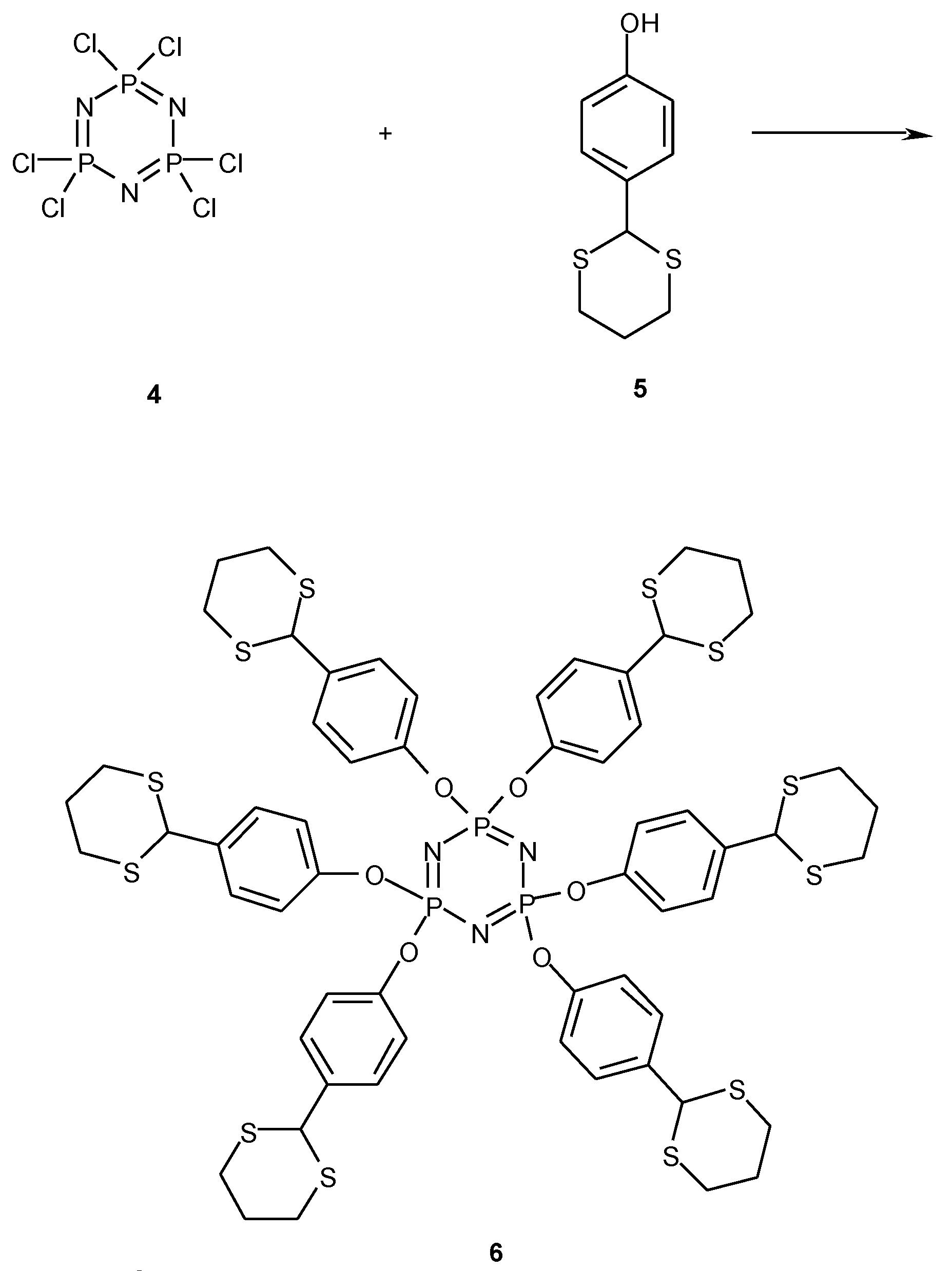
General procedure for preparation of 2-(1,3-dithianyl)-phenols (3a-k)
Hexakis-[p-(1,3-Dithian-2-yl)phenoxy]cyclotriphosphazene (6)
References
- Gassman, G. P.; Drewes, H. R. J. Am. Chem. Soc. 1974, 96, 3003.
- Arai, K.; Oki, M. Bull. Soc. Chem. Jpn. 1976, 49, 553.
- Gassman, G. P.; Amick, R. D. J. Am.. Chem. Soc. 1978, 100, 7611.
- Kruse, C. G.; Wijsman, A.; van der Gen, A. J. Org. Chem. 1979, 44, 1847.
- Allcock, H. R.; Evans, T. L.; Fuller, T. J. Inorg. Chem. 1980, 19, 1026.
- Pond, D. M.; Wang, R. H. S. U.S. 3,936,418 (1976). Chem.Abstr. 1976, 84, 151551. [Google Scholar]
- Munsey, S. M.; Natale, R. N. Heterocycles 1990, 31, 851.
- Dieck, R. L.; Quinn, E. J. (Amstrong Cork Co.) U.S. 4,108,805 (1978). Chem. Abstr. 1979, 90, 72830. [Google Scholar]
- Allcock, H. R.; Smeltz, L. A. J. Am. Chem. Soc. 1976, 98, 4143.
- Ewing, D. F. Org. Mag. Res. 1979, 12, 499.
- Emsley, H.; Udy, P. B. J. Chem. Soc. 1970, 3025.
- Sample Availability: Samples available from the author.
© 1997 MDPI. All rights reserved
Share and Cite
Ladislav, S.; Jozefína, Z.; Nadezda, P. Synthesis of New 2-(1,3-Dithianyl)phenols and Hexakis-[p-(1,3-dithian-2-yl)phenoxy]cyclotriphosphazene. Molecules 1997, 2, 7-10. https://doi.org/10.3390/jan97p3
Ladislav S, Jozefína Z, Nadezda P. Synthesis of New 2-(1,3-Dithianyl)phenols and Hexakis-[p-(1,3-dithian-2-yl)phenoxy]cyclotriphosphazene. Molecules. 1997; 2(1):7-10. https://doi.org/10.3390/jan97p3
Chicago/Turabian StyleLadislav, Stibrányi, Zúziová Jozefína, and Prónayová Nadezda. 1997. "Synthesis of New 2-(1,3-Dithianyl)phenols and Hexakis-[p-(1,3-dithian-2-yl)phenoxy]cyclotriphosphazene" Molecules 2, no. 1: 7-10. https://doi.org/10.3390/jan97p3




