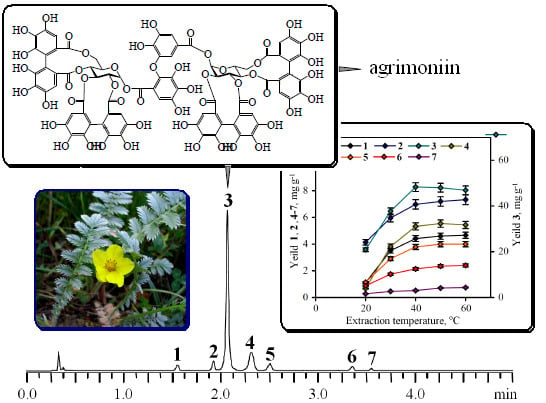
3.3. Extraction and Isolation
The Yakutian sample [PA-Y-02(2013)] of
P. anserina herb (1.64 kg) were air-dried, ground, and extracted with 20 L of 70% acetone [
40] at 40 °C two times (90 min each) on water bath with continuous stirring and the extracts were concentrated under reduced pressure to yield 541 g of crude extract. The crude extract was resuspended in water (1:5,
v/
v) and successively partitioned with hexane and EtOAc. The organic layers were dried
in vacuo to yield 21.1 and 164.6 g of hexane and EtOAc fraction residues respectively. The EtOAc fraction (110 g) was chromatographed over Sephadex LH-20 (8 cm × 90 cm), eluting with 95% ethanol, 80% acetone and 50% acetone to obtain 3 fractions (fr. 1, 14 g; fr. 2, 63 g; fr. 3, 27 g). Fraction 2 (60 g) was re-chromatographed on a Sephadex LH-20 (5 cm × 110 cm), eluting with acetone-water (100:0→0:100) to obtain 11 fractions (frs. 2/1–2/11). Fr. 2/3 was chromatographed on pHPLC (Summit HPLC-system with UV-Vis detector (Dionex, Sunnyvale, CA, USA), column LiChrosorb RP-18 (10 mm × 250 mm, 7 μm, Merck), T 35 °C, flow rate 2 mL/min; solvent, linear gradient of 5%–80% of MeCN in H
2O for 90 min; detector at 270 nm) to give 20 frs. (frs. 2/3-1–2/3-20). Fr. 2/3-11 (t
R 31–34 min) was re-chromatographed in the same conditions to give agrimoniin (
xiii; 3.14 g) [
31]. Fr. 2/5 was chromatographed on pHPLC to give 20 frs. (frs. 2/5-1–2/5-20). Frs. 2/5-6–2/5-7 (t
R 24–29 min) were combined and re-chromatographed (pHPLC) to give agrimonic acid B (
xii; 102 mg) [
31,
40]. Frs. 2/5-8–2/5-9 (t
R 29–31 min) were combined and re-chromatographed (pHPLC) to give agrimonic acid A (
xi; 164 mg) [
31,
40]. Fr. 2/8 was chromatographed on pHPLC to give 20 frs. (frs. 2/8-1–2/8-20). Frs. 2/8-9 (t
R 35–39 min) was re-chromatographed (pHPLC) to give potentillin (
x; 54 mg) [
31]. Fr. 3 (25 g) was subjected on a polyamide column (500 g), eluting with H
2O (12 L), 40% EtOH (21 L) and 90% EtOH (7 L). These elutes were brought dried
in vacuo to yield 1.4, 14.6 and 7.3 g of H
2O (3/1), 40% EtOH (3/2) and 90% EtOH fraction (3/3) residue respectively. Fr. 3/1 (1.2 g) was subjected on an Amberlite XAD1180N column (100 g) preconditioned with 90% ethanol and water, eluting with H
2O (1 L), 40% EtOH (1.5 L) and 90% EtOH (1 L). These elutes were brought dried
in vacuo to yield 655, 308 and 101 mg of H
2O (3/1-1), 40% EtOH (3/1-2) and 90% EtOH fraction (3/1-3) residue respectively. Fr. 3/1-2 (300 mg) was chromatographed on pHPLC (Summit HPLC-system with UV-Vis detector (Dionex, Sunnyvale, CA, USA), column LiChrosorb RP-18 (10 mm × 250 mm, 7 μm, Merck), T 35 °C, flow rate 2 mL/min; solvent, linear gradient of 5%–10% of MeCN in H
2O for 50 min; detector at 316 nm) to give 10 frs. [frs. 3/1-2(1)–3/1-2(10)]. Fr. 3/1-2(3)–3/1-2(5) (t
R 15–22 min) were combined and re-chromatographed in the same conditions to give 2-pyrone-4,6-dicarboxylic acid (
xvii; 52 mg) [
13]. Fr. 3/2 (14 g) was chromatographed over silica column (3 cm × 100 cm), eluting with CHCl
3-MeOH (100:0→0:100) to obtain 11 fractions (frs. 3/2-1–3/2-11). Fr. 3/2-3 was crystallized from MeOH to give reynoutrin (quercetin-3-
O-β-
d-xylopyranoside;
v; 11 mg) [
41]. Frs. 3/2-4–3/2-5 were combined and chromatographed over Sephadex LH-20 (2 cm × 60 cm), eluting with ethanol-water (90:10→10:90) to give miquelianin (quercetin-3-
O-β-
d-glucuronopyranoside;
iv; 129 mg) [
42], ellagic acid (
xvi; 63 mg) [
43] and isoquercitrin (quercetin-3-
O-β-
d-glucopyranoside;
iii; 27 mg) [
44]. Fr. 3/2-6 was chromatographed over Sephadex LH-20 (2 × 60 cm), eluting with ethanol-water (90:10→10:90) to give rutin (quercetin-3-
O-rutinoside;
ii; 29 mg) [
44] and caffeic acid (
xiv; 38 mg) [
45]. Fr. 3/2-9 was chromatographed over Sephadex LH-20 (2 cm × 50 cm), eluting with ethanol-water (90:10→10:90) to myricetin-3-
O-β-
d-glucuronopyranoside (
i; 163 mg) [
46] and 3-
O-caffeoylquinic acid (
xv; 14 mg) [
47]. Frs. 3/3 (7 g) was chromatographed over Sephadex LH-20 (2 cm × 80 cm), eluting with ethanol-water (90:10→10:90) to give 9 fractions (frs. 3/3-1–3/3-9). Fr. 3/3-2 was separated using pTLC (solvent: toluene-EtOAc-HCOOH 5:4:1) to give kaempferol-3-
O-α-
l-rhamnopyranoside (
ix; 37 mg) [
48]. Frs. 3/3-3 and 3/3-4 were combined and chromatographed on pHPLC [Summit HPLC-system with UV-Vis detector (Dionex, Sunnyvale, CA, USA), column LiChrosorb RP-18 (10 × 250 mm, 7 μm, Merck), T 35 °C, flow rate 2 mL/min; solvent, linear gradient of 5%–40% of MeCN in 5% HCOOH/H
2O for 60 min; detector at 350 nm) to give 12 frs. [frs. 3/3-(3-4)-1–3/3-(3-4)-12]. Fr. 3/3-(3-4)-8 (t
R 38–42 min) was crystallized to give isorhamnetin-3-
O-β-
d-glucopyranoside (
vii; 18 mg) [
49]. Fr. 3/3-(3-4)-11 (t
R 49–52 min) was crystallized to give isorhamnetin-3-
O-β-
d-glucuronopyranoside (
viii; 27 mg) [
42]. Frs. 3/5–3/7 were combined and chromatographed over Sephadex LH-20 (1 cm × 50 cm), eluting with ethanol-water (90:10→10:90) to give quercitrin (quercetin-3-
O-α-
l-rhamnopyranoside;
vi; 34 mg) [
50].
Potentillin (x). Off-white powder. tR 2.122 min. UV (λmax) nm 220, 255. (−)ESI-MS m/z 935 [M−H]−. 1H-NMR (500 MHz, MeOH-d4) δ 7.31 (2H, c; H-2', H-6', galloyl), 6.71, 6.62, 6.54, 6.38 (each 1H, c; H-3''', H-3'''', H-3''''', H-3'''''', HHDP), 6.57 (1H, d, J = 3.5 Hz; H-1, α-Glcp), 5.60 (1H, dd, J = 9.1, 10.2 Hz; H-3, α-Glcp), 5.36 (1H, dd, J = 3.5, 9.1 Hz; H-2, α-Glcp), 5.29 (1H, dd, J = 6.0, 12.9 Hz; H-6a, α-Glcp), 5.23 (1H, t, J = 10.2 Hz; H-4, α-Glcp), 4.64 (1H, dd, J = 6.0, 10.2 Hz; H-5, α-Glcp), 3.82 (1H, d, J = 13.0 Hz; H-6b, α-Glcp). 13C-NMR (125 MHz, MeOH-d4) δ 171.7, 169.4, 169.0, 168.5, 168.0 (carbonyls, COO), 146.2 (C-3', C-5', galloyl), 147.0, 146.8, 146.5, 146.1 (C-6'', C-6''', C-6'''', C-6''''', HHDP), 145.9, 145.4 (2C), 145.0 (C-4'', C-4''', C-4'''', C-4''''', HHDP), 140.7 (C-4', galloyl), 137.4 (2C), 137.0, 136.8 (C-5'', C-5''', C-5'''', C-5''''', HHDP), 126.9, 126.5, 126.0, 125.7 (C-2'', C-2''', C-2'''', C-2''''', HHDP), 120.1 (C-1', galloyl), 116.7, 116.5, 115.6, 115.0 (C-1'', C-1''', C-1'''', C-1''''', HHDP), 110.1 (C-2', C-6', galloyl), 108.9, 108.4, 107.9, 107.5 (C-3'', C-3''', C-3'''', C-3''''', HHDP), 90.5 (C-1, α-Glcp), 75.5 (C-3, α-Glcp), 74.0 (C-2, α-Glcp), 70.7 (C-5, α-Glcp), 68.7 (C-4, α-Glcp), 63.0 (C-6, α-Glcp).
Agrimonic acid A (xi). Off-white powder. tR 1.876 min. UV (λmax) nm 205, 274. (−)ESI-MS m/z 1103 [M−H]−. 1H-NMR (500 MHz, MeOH-d4) δ 7.32 (1H, d, J = 2.1 Hz; H-6', DHDG), 7.22 (1H, c; H-6'', DHDG), 6.89 (1H, d, J = 2.2 Hz; H-2', DHDG), 6.72, 6.68, 6.40, 6.32 (each 1H, c; H-3''', H-3'''', H-3''''', H-3'''''', HHDP), 6.55 (1H, d, J = 3.4 Hz; H-1, α-Glcp), 5.58 (1H, dd, J = 9.2, 10.1 Hz; H-3, α-Glcp), 5.34 (1H, dd, J = 3.4, 9.2 Hz; H-2, α-Glcp), 5.27 (1H, dd, J = 5.9, 12.9 Hz; H-6a, α-Glcp), 5.20 (1H, t, J = 10.1 Hz; H-4, α-Glcp), 4.61 (1H, dd, J = 5.9, 10.1 Hz; H-5, α-Glcp), 3.80 (1H, d, J = 13.1 Hz; H-6b, α-Glcp). 13C-NMR (125 MHz, MeOH-d4) δ 171.9, 169.9, 169.7, 169.2, 168.5, 168.2 (carbonyls, COO), 150.1 (C-3', DHDG), 147.7 (C-5', DHDG), 147.3, 147.1 (2C), 146.9 (C-6''', C-6'''', C-6''''', C-6'''''', HHDP), 145.7, 145.5, 145.2 (2C) (C-4''', C-4'''', C-4''''', C-4'''''', HHDP), 143.4 (C-5'', DHDG), 142.8 (C-3'', DHDG), 142.6 (C-4', DHDG), 141.3 (C-4'', DHDG), 138.1 (C-2'', DHDG), 137.8 (2C), 137.5 (2C) (C-5''', C-5'''', C-5''''', C-5'''''', HHDP), 127.7, 127.5, 127.1, 126.9 (C-2''', C-2'''', C-2''''', C-2'''''', HHDP), 120.9 (C-1', DHDG), 117.0 (C-1'', DHDG), 116.9, 116.8, 116.0, 115.4 (C-1''', C-1'''', C-1''''', C-1'''''', HHDP), 112.9 (C-2', DHDG), 110.8 (C-6'', DHDG), 109.3 (2C), 109.1, 108.9 (C-3''', C-3'''', C-3''''', C-3'''''', HHDP), 108.6 (C-6', DHDG), 90.9 (C-1, α-Glcp), 75.9 (C-3, α-Glcp), 74.2 (C-2, α-Glcp), 71.0 (C-5, α-Glcp), 69.2 (C-4, α-Glcp), 63.5 (C-6, α-Glcp).
Agrimonic acid B (xii). Off-white powder. tR 1.563 min. UV (λmax) nm 205, 274. (−)ESI-MS m/z 1103 [M−H]−. 1H-NMR (500 MHz, MeOH-d4) δ 7.29 (1H, d, J = 2.1 Hz; H-6', DHDG), 7.24 (1H, c; H-6'', DHDG), 6.84 (1H, d, J = 2.2 Hz; H-2', DHDG), 6.74, 6.70, 6.44, 6.35 (each 1H, c; H-3''', H-3'''', H-3''''', H-3'''''', HHDP), 6.51 (1H, d, J = 3.4 Hz; H-1, α-Glcp), 5.52 (1H, dd, J = 9.2, 10.1 Hz; H-3, α-Glcp), 5.33 (1H, dd, J = 3.4, 9.2 Hz; H-2, α-Glcp), 5.21 (1H, dd, J = 5.9, 12.9 Hz; H-6a, α-Glcp), 5.18 (1H, t, J = 10.1 Hz; H-4, α-Glcp), 4.58 (1H, dd, J = 5.9, 10.1 Hz; H-5, α-Glcp), 3.67 (1H, d, J = 13.1 Hz; H-6b, α-Glcp). 13C-NMR (125 MHz, MeOH-d4) δ 171.7, 170.3, 169.9, 169.2, 168.6, 168.0 (carbonyls, COO), 149.9 (C-3'', DHDG), 147.5 (C-5', DHDG), 147.1, 147.2 (2C), 146.7 (C-6''', C-6'''', C-6''''', C-6'''''', HHDP), 145.8, 145.4 (2C), 145.1 (C-4''', C-4'''', C-4''''', C-4'''''', HHDP), 143.8 (C-5'', DHDG), 143.1 (C-3', DHDG), 142.8 (C-4'', DHDG), 141.5 (C-4', DHDG), 138.0, 137.9, 137.6, 137.4 (C-5''', C-5'''', C-5''''', C-5'''''', HHDP), 136.9 (C-2′, DHDG), 127.8, 127.5, 127.0, 126.7 (C-2''', C-2'''', C-2''''', C-2'''''', HHDP), 121.4 (C-1', DHDG), 117.2 (C-1'', DHDG), 116.6, 116.7, 115.9, 115.2 (C-1''', C-1'''', C-1''''', C-1'''''', HHDP), 112.4 (C-2'', DHDG), 110.7 (C-6'', DHDG), 109.4, 109.2, 109.0, 108.7 (C-3''', C-3'''', C-3''''', C-3'''''', HHDP), 108.4 (C-6′, DHDG), 90.5 (C-1, α-Glcp), 76.2 (C-3, α-Glcp), 74.6 (C-2, α-Glcp), 71.5 (C-5, α-Glcp), 68.8 (C-4, α-Glcp), 63.2 (C-6, α-Glcp).
Agrimoniin (xiii). Off-white powder. tR 2.063 min. UV (λmax) nm 230, 268. (−)ESI-MS m/z 1869 [M−H]−. 1H-NMR (500 MHz, MeOH-d4) δ 7.34 (1H, d, J = 2.1 Hz; H-6'', DHDG), 7.20 (1H, c; H-6''', DHDG), 6.91 (1H, d, J = 2.1 Hz; H-2'', DHDG), 6.73, 6.70, 6.65, 6.62, 6.60, 6.58, 6.51, 6.46 (each 1H, c; H-3'''', H-3''''', H-3'''''', H-3''''''', H-3'''''''', H-3''''''''', H-3'''''''''', H-3''''''''''', HHDP), 6.56 (1H, d, J = 4.0 Hz; H-1, α-Glcp-1), 6.51 (1H, d, J = 4.0 Hz; H-1', α-Glcp-2), 5.52 (1H, dd, J = 9.6, 10.2 Hz; H-3', α-Glcp-2), 5.48 (1H, dd, J = 9.6, 10.2 Hz; H-3, α-Glcp-1), 5.38 (1H, dd, J = 4.0, 9.5 Hz; H-2', α-Glcp-2), 5.32 (1H, dd, J = 4.0, 9.5 Hz; H-2, α-Glcp-1), 5.29 (1H, dd, J = 6.1, 13.0 Hz; H-6'a, α-Glcp-2), 5.22 (1H, dd, J = 6.0, 13.0 Hz; H-6a, α-Glcp-1), 5.20 (1H, t, J = 9.5 Hz; H-4', α-Glcp-2), 5.14 (1H, t, J = 9.5 Hz; H-4, α-Glcp-1), 4.61 (1H, dd, J = 6.1, 10.2 Hz; H-5', α-Glcp-2), 4.47 (1H, dd, J = 6.1, 10.2 Hz; H-5, α-Glcp-1), 3.70 (1H, d, J = 13.6 Hz; H-6'b, α-Glcp-2), 3.65 (1H, d, J = 13.6 Hz; H-6b, α-Glcp-1). 13C-NMR (125 MHz, MeOH-d4) δ 171.4, 171.0, 169.7 (2C), 169.5, 169.3, 169.0 (2C), 166.6, 166.3 (carbonyls, COO), 150.1 (C-3'', DHDG), 147.9 (C-5'', DHDG), 147.5 (4C), 147.1, 146.8 (2C), 146.5 (C-6'''', C-6''''', C-6'''''', C-6''''''', C-6'''''''', C-6''''''''', C-6'''''''''', C-6''''''''''', HHDP), 146.0, 145.8, 145.5 (3C), 145.1, 144.7, 144.3 (C-4'''', C-4''''', C-4'''''', C-4''''''', C-4'''''''', C-4''''''''' C-4'''''''''', C-4''''''''''', HHDP), 143.4 (C-5''', DHDG), 142.8 (C-3''', DHDG), 142.6 (C-4'', DHDG), 141.3 (C-4''', DHDG), 138.1 (C-2''', DHDG), 137.6 (2C), 137.3, 136.8, 136.5 (3C), 136.2 (C-5'''', C-5''''', C-5'''''', C-5''''''', C-5'''''''', C-5''''''''', C-5'''''''''', C-5''''''''''', HHDP), 126.7, 126.4, 126.2, 126.0 (2C), 125.7, 125.4 (2C) (C-2'''', C-2''''', C-2'''''', C-2''''''', C-2'''''''', C-2''''''''', C-2'''''''''', C-2''''''''''', HHDP), 120.9 (C-1'', DHDG), 117.0 (C-1''', DHDG), 116.7, 116.5 (2C), 116.0, 115.8, 115.6 (2C), 115.3 (C-1'''', C-1''''', C-1'''''', C-1''''''', C-1'''''''', C-1''''''''', C-1'''''''''', C-1''''''''''', HHDP), 112.9 (C-2'', DHDG), 110.8 (C-6''', DHDG), 109.1, 108.7 (4C), 108.5, 108.3 (2C) (C-3'''', C-3''''', C-3'''''', C-3''''''', C-3'''''''', C-3''''''''', C-3'''''''''', C-3''''''''''', HHDP), 108.0 (C-6'', DHDG), 90.7 (C-1, α-Glcp-1), 91.2 (C-1', α-Glcp-2), 76.0 (C-3', α-Glcp-2), 75.6 (C-3, α-Glcp-1), 74.4 (C-2, α-Glcp-1), 74.1 (C-2', α-Glcp-2), 71.4 (C-5', α-Glcp-2), 71.0 (C-5, α-Glcp-1), 68.9 (C-4, α-Glcp-1), 68.5 (C-4', α-Glcp-2), 63.3 (C-6, α-Glcp-1), 63.1 (C-6', α-Glcp-2).