Preparation, Cell Compatibility and Degradability of Collagen-Modified Poly(lactic acid)
Abstract
:1. Introduction

2. Results and Discussion
2.1. Preparation of Collagen-PLA
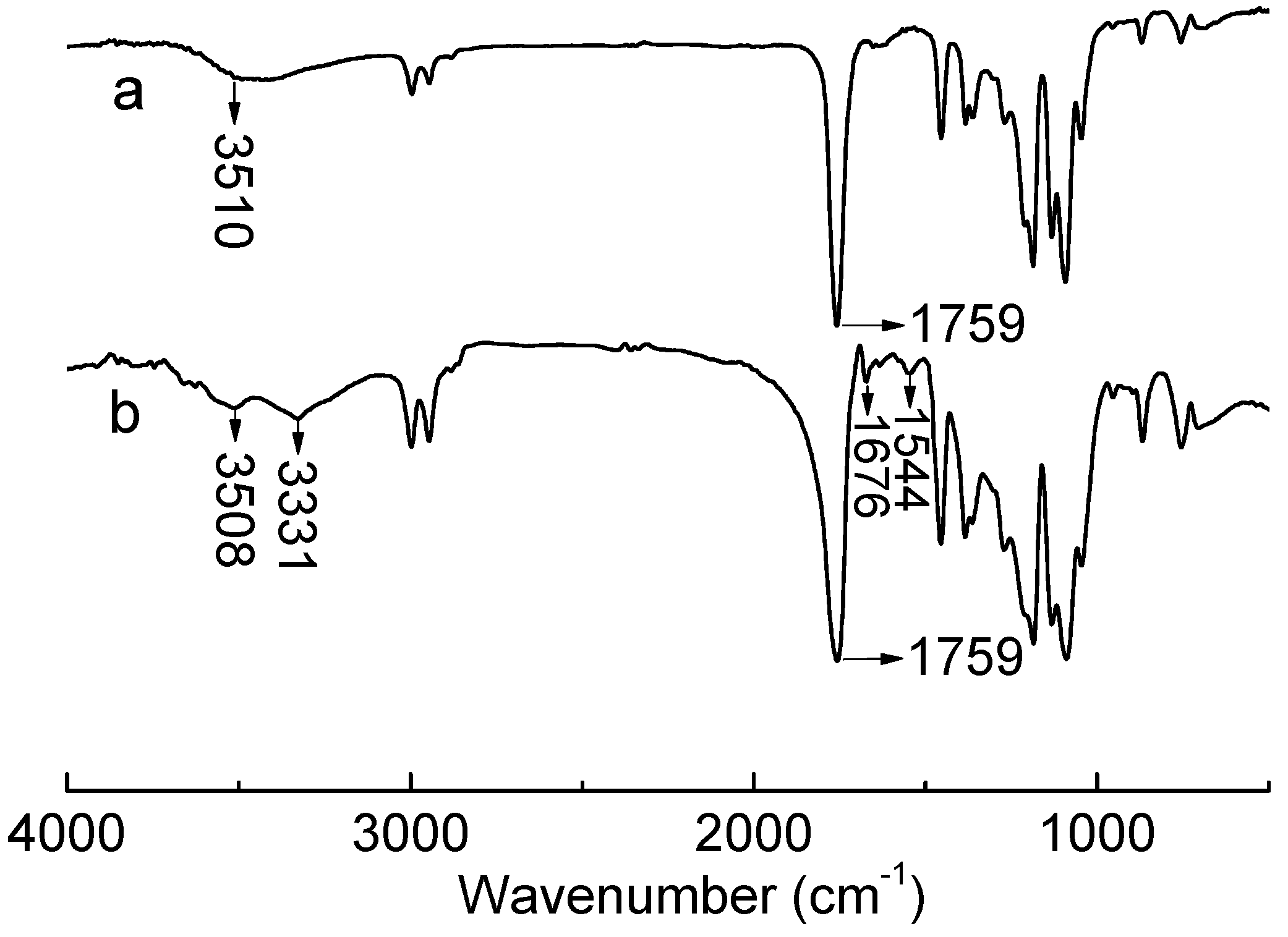

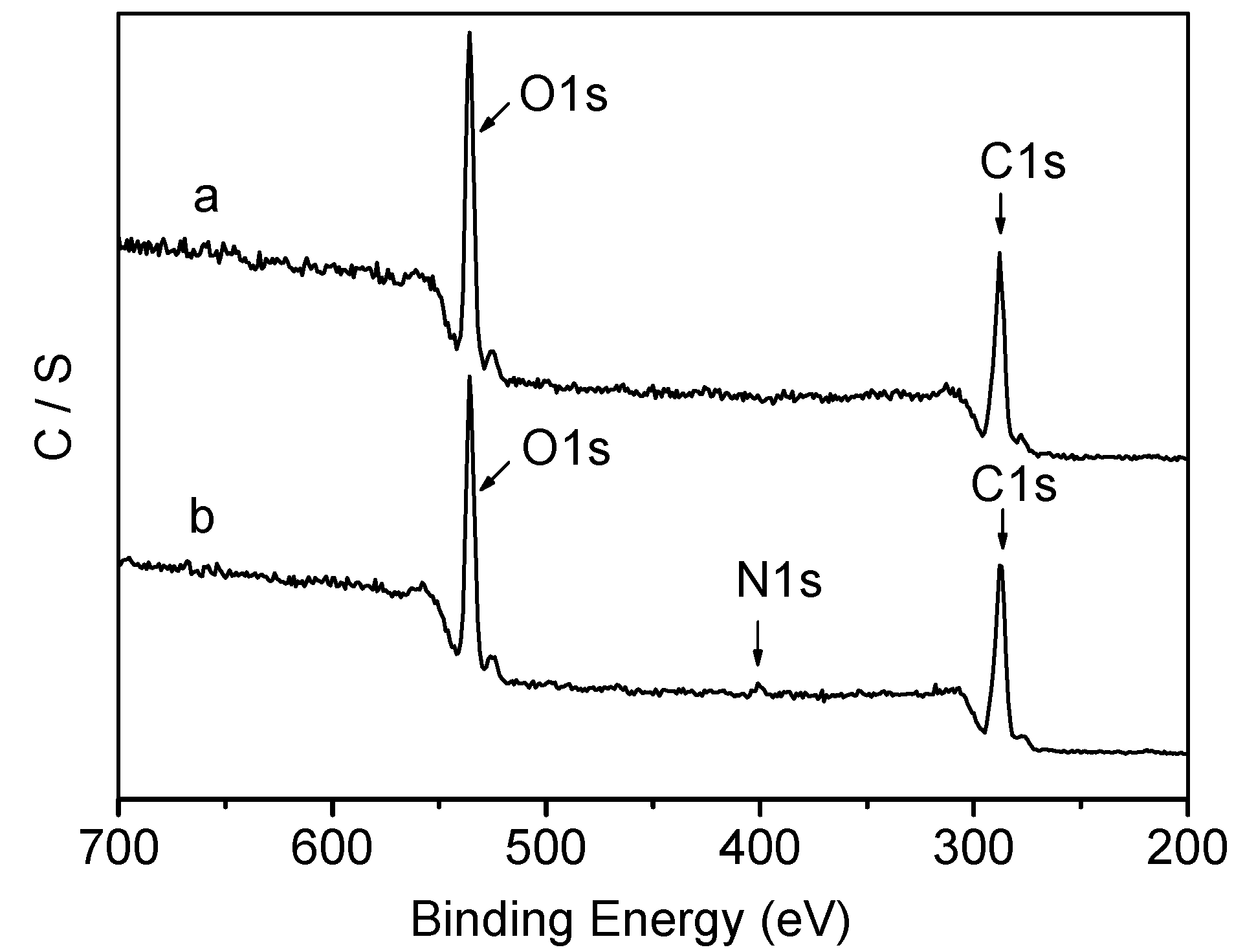
2.2. Thermal Properties

2.3. Graft Ratio of Collagen and Relative Molecular Mass
2.4. Hydrophilicity

2.5. Cell Compatibility


2.6. Degradability


3. Experimental Section
3.1. Materials
3.2. Preparation of Collagen-PLA
3.3. Characterization
3.4. Hydrophilicity and Degradability Test
3.5. Cell Compatibility Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kulkarni, R.K.; Pani, K.C.; Neuman, C. Poly (lactic acid) for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Tao, A.J.; Li, H.Y. Complex membrane gluided periodontal tissue regeneration. J. Oral Sci. Res. 2009, 25, 298–301. [Google Scholar]
- Zhang, J.; Mei, F.; Cai, Q. Human Periodontal Ligament Cells Cultured on Lattice-like and Non-woven Electrospun Poly(L-lactide) Nanofiber Scaffolds in vitro. Chin. J. Biomed. Eng. 2009, 28, 754–759. [Google Scholar]
- Liu, B.; Chen, P.; Zang, X.X. Study of planting AnHAC/PLA to promote healing of tooth extraction wound and decrease alveolar crest absorption. Stomatology 2010, 30, 38–41. [Google Scholar]
- Zhuo, R.M.; Yin, C.; Wu, Y.N.; Zhu, L.; Zheng, S.Q. Synthesis and Degradation Behavior of Poly (DL-lactide) for Ophthalmic Application. Appl. Chem. 1997, 14, 102–104. [Google Scholar]
- Li, Y.; Wu, X.Y.; Qiao, Z. Experimental research of prevention of scar formation after trabeculectomy by several kinds of PLA. Shandong Med. J. 2005, 45, 9–12. [Google Scholar]
- Liu, D.Y.; Ma, J.X.; Cao, D.Y. Sustained release ability and safety of matrine polyactic acid microsphere intravitreal injection. Chin. Ophthalmic Res. 2010, 28, 34–38. [Google Scholar]
- Ashammakhi, N.; Rokkanen, P. Absorbable polyglycolide devices in trauma and bone surgery. Biomaterials 1997, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bisson, I.; Kosinski, M.; Ruault, S. Acrylic acid grafting and collagen immobilization on poly (ethylene terephthalate) surfaces for adherence and growth of human bladder smooth muscle cells. Biomaterials 2002, 23, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Flenuning, R.G.; Murphy, C.J.; Abrams, G.A. Effects of syntheticmicro-and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J. Bone formation by three-dimensiona stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ishaug-Riley, S.L.; Crane-Knlge, G.M.; Yaszemski, M.L. Threedimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 1998, 19, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, H.; Yang, F. Preparation and cell affinity of microtubular orientation-structured PLGA (70/30) blood vessel scaffold. Biomaterials 2008, 29, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.Z.; Zha, L.S. Preparation, characterization and application of pyrene-loaded methoxy poly (ethylene glycol)-poly (lactic acid) copolymer nanoparticles. Colloid Polym. Sci. 2004, 282, 1323–1328. [Google Scholar] [CrossRef]
- Manca, M.L.; Loy, G.; Zaru, M. Release of rifampicin from chitosan, PLGA and chitosan-coated PLGA microparticles. Colloids Surf. B Biointerfaces 2008, 67, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Kan, B.; Gou, M.L. Preparation of MPEG-PLA nanoparticle for honokiol delivery in vitro. Int. J. Pharm. 2010, 386, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.H.; Han, Y.R. Novel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogels. Int. J. Pharm. 2010, 386, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.F.; Balakrishnan, P.; Cui, F.D. The effects of mixed MPEGPLA/Pluronic R copolymermicelles on the bioavailability and multidrug resistance of docetaxel. Biomaterials 2010, 31, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Su, S.R.; Liu, L.L.; Li, X.; Cui, M.M.; Ma, F. Study on Synthesis and Application of Collagen Modified Polylactic Acid. Polym. Compos. 2014, 36, 88–93. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Xu, Y.Z.; Lu, J.; Zhu, X.; Fan, H.S.; Zhang, X.D. Preparation and characterization of collagen-modified polylactide microparticles. Mater. Lett. 2007, 61, 2601–2605. [Google Scholar] [CrossRef]
- Gutierrez-Villarreal, M.H.; Ulloa-Hinojosa, M.G.; Gaona-Lozano, J.G. Surface functionalization of poly (lactic acid) film by UV-photografting of N-vinylpyrrolidone. J. Appl. Polym. Sci. 2008, 110, 163–169. [Google Scholar] [CrossRef]
- Bae, G.Y.; Jang, J.; Jeong, Y.G. Superhydrophobic PLA fabrics prepared by UV photo-grafting of hydrophobic silica particles possessing vinyl groups. J. Colloid Interface Sci. 2010, 344, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gao, C.Y.; Xie, Y.; Gong, Y.H.; Shen, J.C. Collagen-coated polyactide microspheres as chondrocyte microcarriers. Biomaterials 2005, 26, 6305–6313. [Google Scholar] [CrossRef] [PubMed]
- Steffens, G.C.M.; Nothdurft, L.; Buse, G.; Thissen, H.; Höcker, H.; Klee, D. High density binding of proteins and peptides to poly(D,L-lactide) grafted with polyacrylic acid. Biomaterials 2002, 23, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.N.; Fan, Y.G.; Ma, J.B. Synthesis of a biodegradable tadpole-shaped polymer via the coupling reaction of polylactide onto mono(6-(2-aminoethyl)amino-6-deoxy)-β-cyclodextrin and its properties as the new carrier of protein delivery system. J. Control. Release 2005, 107, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Yu, H.Z.; Cao, Z.Y. Chemical modification of β-cyclodextrin chitosan and its adsorption on phenol, n-nonylphenol and resorcinol. J. Cent. South Univ. 2007, 38, 112–115. [Google Scholar]
- Dong, H.Q.; Xu, Q.; Li, Y.Y. The synthesis of biodegradable graft copolymer cellulose-graft-poly (L-lactide) and the study of its controlled drug release. Colloid Surf. B 2008, 66, 26–33. [Google Scholar] [CrossRef]
- Bang, J.Y.; Song, C.E.; Kim, C. Cytotoxicity of amphotericin B-incorporated polymeric micelles composed of poly(DL-lactide-co-glycolide)/dextran graft copolymer. Arch. Pharm. Res. 2008, 31, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.L.; Liu, C.; Chen, W. Synthesis and characterization of chitosan grafted oligo (l-lactic acid). Macromol. Biosci. 2003, 3, 653–662. [Google Scholar] [CrossRef]
- Li, X.X.; Voll, C.; Kissel, T. Biodegradable brush-like graft polymers from poly(d,l-Lactide) or poly(d,l-Lactide-co-glycolide) and charge-modified, hydrophilic dextrans as backbone in vitro degradation and controlled releases of hydrophilic macromolecules. Polymer 1998, 39, 3087–3095. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, C.P.; Lee, Y.D. Preparation of amphiphilic poly(L-lactide)-graft-chondroitin sulfate copolymer self-aggregates and its aggregation behavior. Biomacromolecules 2006, 7, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, F. Collagen-biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grande, D.A.; Halberstadt, C.; Naughton, G. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mat. Res. 1997, 34, 211–220. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yang, P.F.; Li, P.; Xin, J.J.; Su, R.X. Synthesis of collagen-modified polylactide and its application in drug delivery. J. Appl. Polym. Sci. 2013, 129, 3290–3296. [Google Scholar] [CrossRef]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of blending calcium compounds on hydrolytic degradation of poly(dl-lactic acid-co-glycolic acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.I.; Lee, C.W.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Melt/solid polycondensation of l-lactic acid: An alternative route to poly(l-lactic acid) with high molecular weight. Polymer 2001, 42, 5059–5062. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, Z.Y.; Wang, J.; Mai, H.Z.; Yan, B.; Yang, F. Direct synthesis of poly(D,L-lactic acid) by melt polycondensation and its application in drug delivery. J. Appl. Polym. Sci. 2004, 91, 2143–2150. [Google Scholar] [CrossRef]
- Zheng, J.; Nothrup, S.; Hornsby, P.J. Modification of materials formed from poly(L-lactic acid) to enable covalent binding of biopolymers: Application to high-density three-dimensional cell culture in foams with attached collagen. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Grath, M.R. Protein measurement by ninhydrin determination of amino acids released by alkaline hydrolysis. Anal. Biochem. 1972, 49, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the collagen-modified PLA are avaiable from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Liu, L.; Guo, N.; Su, R.; Ma, F. Preparation, Cell Compatibility and Degradability of Collagen-Modified Poly(lactic acid). Molecules 2015, 20, 595-607. https://doi.org/10.3390/molecules20010595
Cui M, Liu L, Guo N, Su R, Ma F. Preparation, Cell Compatibility and Degradability of Collagen-Modified Poly(lactic acid). Molecules. 2015; 20(1):595-607. https://doi.org/10.3390/molecules20010595
Chicago/Turabian StyleCui, Miaomiao, Leili Liu, Ning Guo, Ruixia Su, and Feng Ma. 2015. "Preparation, Cell Compatibility and Degradability of Collagen-Modified Poly(lactic acid)" Molecules 20, no. 1: 595-607. https://doi.org/10.3390/molecules20010595




