Synthesis, Fluorescence Properties, and Antiproliferative Potential of Several 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Derivatives
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

2.2. In Vitro Biological Activity
2.2.1. Antiproliferative Activities of the Target Compounds against A549 and NCI-H460 Cells in Vitro


| Compound | R | IC50 ± SD (μM) a,b | |
|---|---|---|---|
| A549 | NCI-H460 | ||
| 5a | 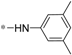 | >60 | 46.41 ± 4.22 |
| 5b | 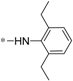 | >60 | >60 |
| 5c | 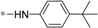 | >60 | >60 |
| 5d | 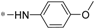 | 38.71 ± 3.62 | 44.39 ± 2.98 |
| 5e | 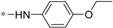 | 20.53 ± 1.84 | 29.19 ± 2.61 |
| 5f | 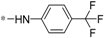 | 29.72 ± 3.85 | >60 |
| 5g |  | >60 | >60 |
| 5h | 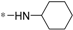 | >60 | >60 |
| 5i | 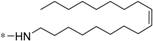 | >60 | >60 |
| 6a | 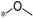 | >60 | >60 |
| 6b | 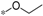 | >60 | >60 |
| 6c |  | >60 | >60 |
| 6d |  | >60 | >60 |
| 6e |  | >60 | >60 |
| 6f | 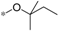 | >60 | >60 |
| 6g |  | >60 | >60 |
| 7-HC | — | 33.82 ± 2.62 | 38.55 ± 2.91 |
| Cisplatin | — | 15.42 ± 1.06 | 9.61 ± 0.83 |
2.2.2. Apoptosis Induced by Compound 5e

| Group | Conc. b (µM) | 48 h Incubation | 72 h Incubation | ||||
|---|---|---|---|---|---|---|---|
| Viable Cells (Q1, %) | Apoptotic Cells (Q2 + Q3, %) | Necrotic Cells (Q4, %) | Q1 (%) | Q2 + Q3 (%) | Q4 (%) | ||
| Control | 0 | 94.7 ± 2.6 | 4.72 ± 0.41 | 0.62 ± 0.19 | 91.8 ± 3.3 | 7.22 ± 0.52 | 0.97 ± 0.13 |
| 7-HC | 20 | 85.7 ± 2.8 | 11.3 ± 1.4 ** | 3.04 ± 0.29 | 81.1 ± 2.4 | 14.2 ± 1.9 * | 4.71 ± 0.49 |
| Compd. 5e | 10 | 91.4 ± 3.1 | 6.51 ± 1.31 | 2.04 ± 0.31 | 85.3 ± 2.9 | 10.9 ± 2.1 | 3.77 ± 0.31 |
| Compd. 5e | 20 | 80.7 ± 2.5 | 13.9 ± 2.2 ** | 5.41 ± 0.62 | 73.9 ± 2.7 | 19.1 ± 1.4 ** | 6.98 ± 0.42 |
2.2.3. Cell Cycle Analysis

| Group | Conc. (µM) | G0/G1 Phase (%) | S Phase (%) | G2/M Phase (%) |
|---|---|---|---|---|
| Control | 0 | 70.71 ± 2.79 | 16.79 ± 1.37 | 12.50 ± 1.41 |
| 7-HC | 20 | 79.83 ± 2.39 * | 8.38 ± 0.44 | 11.79 ± 1.69 |
| Compd. 5e | 10 | 72.62 ± 2.67 | 15.77 ± 0.84 | 11.60 ± 1.31 |
| Compd. 5e | 20 | 80.52 ± 2.29 * | 13.14 ± 1.01 | 6.31 ± 0.62 |
| Compd. 5e | 30 | 85.51 ± 2.03 * | 8.82 ± 0.91 | 5.67 ± 0.43 |
2.2.4. Induced ROS Generation by Compound 5e

2.3. Absorption Spectra, Fluorescence Spectra, and Living Cell Imaging


| Entry | Compound | λabs, nm a | λem, nm a | Stokes Shift b | Φf | |
|---|---|---|---|---|---|---|
| 1 | 5c | 383 | 442 | 3485 cm−1 | 59 nm | <0.1 |
| 2 | 5e | 383 | 441 | 3433 cm−1 | 58 nm | <0.1 |
| 3 | 5f | 381 | 440 | 3519 cm−1 | 59 nm | <0.1 |
| 4 | 5g | 370 | 434 | 3986 cm−1 | 64 nm | 0.49 |
| 5 | 6a | 375 | 437 | 3783 cm−1 | 62 nm | 0.44 |
| 6 | 6b | 375 | 437 | 3783 cm−1 | 62 nm | 0.51 |
| 7 | 6c | 375 | 437 | 3783 cm−1 | 62 nm | 0.58 |
| 8 | 6d | 375 | 437 | 3783 cm−1 | 62 nm | 0.52 |
| 9 | 6e | 375 | 437 | 3783 cm−1 | 62 nm | 0.60 |
| 10 | 6f | 375 | 437 | 3783 cm−1 | 62 nm | 0.62 |
| 11 | 6g | 380 | 441 | 3640 cm−1 | 61 nm | 0.66 |
| 12 | 7-HC | 319 | 396 | 6095 cm−1 | 71 nm | <0.1 |
| 13 | QS c | 348 | 445 | 6264 cm−1 | 97 nm | 0.54 |

3. Experimental Section
3.1. Chemistry
3.1.1. Material and Reagents
3.1.2. Synthesis of 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid (3)
3.1.3. General Procedures for the Preparation of 3-Oxo-3H-benzo[f]chromene-2-amides 5a–i and 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Esters 6a–g
3.2. Biological Activity
3.2.1. Cell Antiproliferative Activity Assay
3.2.2. Apoptosis Detection by Flow Cytometry
3.2.3. Cell Cycle Distribution Analysis
3.2.4. Determination of Intracellular ROS Production
3.3. UV-Vis Absorption and Fluorescence Experiments
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monti, M.; Pinotti, M.; Appendino, G.; Dallocchio, F.; Bellini, T.; Antognoni, F.; Poli, F.; Bernardi, F. Characterization of anti-coagulant properties of prenylated coumarin ferulenol. Biochim. Biophys. Acta 2007, 1770, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.S.; Paul, S.; Mandal, S.K.; Banerjee, A.; Boujedaini, N.; Khuda-Bukhsh, A.R. A synthetic coumarin (4-methyl-7-hydroxycoumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur. J. Pharmacol. 2009, 614, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.M.; Damu, G.L.; Zhou, C. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kudo, E.; Taura, M.; Matsuda, K.; Shimamoto, M.; Kariya, R.; Goto, H.; Hattori, S.; Kimura, S.; Okada, S. Inhibition of HIV-1 replication by a tricyclic coumarin GUT-70 in acutely and chronically infected cells. Bioorg. Med. Chem. Lett. 2013, 23, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [PubMed]
- Chen, X.; Pi, R.; Zou, Y.; Liu, M.; Ma, X.; Jiang, Y.; Mao, X.; Hu, X. Attenuation of experimental autoimmune encephalomyelitis in C57 BL/6 mice by osthole, a natural coumarin. Eur. J. Pharmacol. 2010, 629, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Ellis, E.M. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Shivashankar, K.; Kulkarni, M.V.; Rasal, V.P.; Patel, H.; Mutha, S.S.; Mohite, A.A. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem. 2010, 45, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- El-Haggar, R.; Al-Wabli, R.I. Anti-inflammatory screening and molecular modeling of some novel coumarin derivatives. Molecules 2015, 20, 5374–5391. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kumar, S.; Kaushik, P.; Kaushik, D.; Gupta, G.K. Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-yl)-4-(coumarin-3-yl)thiazoles. Eur. J. Med. Chem. 2013, 62, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, M.E.; Kimpe, D.N.; Moglioni, A.; Vázquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Liu, L.H.; Chen, S.; Cheng, H.; Chen, C.S.; Li, Z.Y.; Qin, S.Y.; Zhang, X.Z. A coumarin derivative as a fluorogenic glycoproteomic probe for biological imaging. Chem. Commun. 2014, 50, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Fang, Y.; Zhao, Y.; Zhao, H.; Wang, Y.; Gu, Y.; Wu, F. Synthesis and in vitro photocytotoxicity of coumarin derivatives for one- and two-photon excited photodynamic therapy. J. Med. Chem. 2013, 56, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhou, L.; Wang, L.; Meng, S.; Gong, A.; Du, F.; Zhang, C. A coumarin-based fluorescent probe for biological thiols and its application for living cell imaging. Org. Biomol. Chem. 2013, 11, 8214–8220. [Google Scholar] [CrossRef]
- Xiao, J.M.; Feng, L.; Zhou, L.S.; Gao, H.Z.; Zhang, Y.L.; Yang, K.W. Novel fluorescent cephalosporins: Synthesis, antimicrobial activity and photodynamic inactivation of antibiotic resistant bacteria. Eur. J. Med. Chem. 2013, 59, 150–159. [Google Scholar] [CrossRef]
- Song, A.; Wang, X.; Lam, K.S. A convenient synthesis of coumarin-3-carboxylic acids via Knoevenagel condensation of Meldrum’s acid with ortho-hydroxyaryl aldehydes or ketones. Tetrahedron Lett. 2003, 44, 1755–1758. [Google Scholar] [CrossRef]
- Gao, W.T.; Hou, W.D.; Zheng, M.R.; Tang, L.J. Clean and convenient one-pot synthesis of 4-hydroxycoumarin and 4-hydroxy-2-quinolinone derivatives. Synth. Commun. 2010, 40, 732–738. [Google Scholar] [CrossRef]
- Scott, J.L.; Raston, C.L. Solvent-free synthesis of 3-carboxycoumarins. Green Chem. 2000, 2, 245–247. [Google Scholar] [CrossRef]
- Bahador, K.; Mahnaz, F.; Saeed, K. Rapid Synthesis of novel and known coumarin-3-carboxylic acids using stannous chloride dihydrate under solvent-free conditions. Helv. Chim. Acta 2012, 95, 455–460. [Google Scholar]
- Garino, C.; Tomita, T.; Pietrancosta, N.; Laras, Y.; Rosas, R.; Herbette, G.; Maigret, B.; Quéléver, G.; Iwatsubo, T.; Kraus, J.L. Naphthyl and coumarinyl biarylpiperazine derivatives as highly potent human β-secretase inhibitors. Design, synthesis, and enzymatic BACE-1 and cell assays. J. Med. Chem. 2006, 49, 4275–4285. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H.; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yan, X.; Zhang, G.; Zhao, W.; Jiao, S. The predictive value of ERCC1 and p53 for the effect of panobinostat and cisplatin combination treatment in NSCLC. Oncotarget 2015, 6, 18997–19005. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.F.; Carus, A.; Meldgaard, P. Intravenous or oral administration of vinorelbine in adjuvant chemotherapy with cisplatin and vinorelbine for resected NSCLC. Lung Cancer 2015, 88, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Dhaheri, A.Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.; Eid, A.; Iratni, R. Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Ahipa, T.N.; Adhikari, A.V. New cyanopyridone based luminescent liquid crystalline materials: Synthesis and characterization. Photochem. Photobiol. Sci. 2014, 13, 1496–1508. [Google Scholar]
- Nizomov, N.; Kholov, A.U.; Ishchenko, A.A.; Ishchenko, V.V.; Khilya, V.P. Electronic structure and spectral fluorescence properties of umbelliferone and herniarin. J. Appl. Spectrosc. 2007, 74, 626–634. [Google Scholar]
- Irvine, M.W.; Costa, B.M.; Volianskis, A.; Fang, G.; Ceolin, L.; Collingridge, G.L.; Monaghan, D.T.; Jane, D.E. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-d-aspartate receptors. Neurochem. Int. 2012, 61, 593–600. [Google Scholar]
- Ukhov, S.V.; Syropyatov, B.Y.; Novikova, V.V.; Balabash, V.A.; Shustov, A.S.; Odegova, T.F. Synthesis and biological activity of substituted 2-iminobenzo[f]coumarin-3-carboxylic acid amides. Pharm. Chem. J. 2011, 45, 228–230. [Google Scholar]
- El-Sharief, A.M.S.; Bedair, A.H.; Aly, F.M.; Mourad, A.E.; El-Agrody, A.M. Some reactions with 3-carbethoxy-5,6-benzocoumarin and related compounds. Egypt. J. Chem. 1983, 25, 41–51. [Google Scholar]
- He, X.W.; Shang, Y.J.; Zhou, Y.; Yu, Z.Y.; Han, G.; Jin, W.J.; Chen, J.J. Synthesis of coumarin-3-carboxylic esters via FeCl3-catalyzed multicomponent reaction of salicylaldehydes, Meldrum’s acid and alcohols. Tetrahedron 2015, 71, 863–868. [Google Scholar] [CrossRef]
- Wang, Z.X.; Peng, M.S.; Song, X.P.; Wang, F. Study on synthesis and fluorescent properties of alkyl benzocoumarin-3-carboxylates. J. Anhui Agric. Sci. 2011, 39, 6314–6315, 6344. [Google Scholar]
- Wang, H.M.; Zhang, L.; Liu, J.; Yang, Z.L.; Zhao, H.Y.; Yang, Y.; Shen, D.; Lu, K.; Fan, Z.C.; Yao, Q.W.; et al. Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. Eur. J. Med. Chem. 2015, 92, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xin, Z.; Wan, Y.; Li, J.; Ye, B.; Xue, X. Synthesis and anticancer activity of some novel indolo[3,2-b]andrographolide derivatives as apoptosis-inducing agents. Eur. J. Med. Chem. 2015, 90, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Sarma, M.D.; Ghosh, R.; Patra, A.; Hazra, B. Synthesis of novel aminoquinonoid analogues of diospyrin and evaluation of their inhibitory activity against murine and human cancer cells. Eur. J. Med. Chem. 2008, 43, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 5e, 5g, and 6g are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.-B.; Wang, X.-F.; Chen, J.-N.; Wu, D.-W.; Li, T.; Shen, X.-C.; Qin, J.-K. Synthesis, Fluorescence Properties, and Antiproliferative Potential of Several 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Derivatives. Molecules 2015, 20, 18565-18584. https://doi.org/10.3390/molecules201018565
Fu X-B, Wang X-F, Chen J-N, Wu D-W, Li T, Shen X-C, Qin J-K. Synthesis, Fluorescence Properties, and Antiproliferative Potential of Several 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Derivatives. Molecules. 2015; 20(10):18565-18584. https://doi.org/10.3390/molecules201018565
Chicago/Turabian StyleFu, Xiao-Bo, Xian-Fu Wang, Jia-Nian Chen, De-Wen Wu, Ting Li, Xing-Can Shen, and Jiang-Ke Qin. 2015. "Synthesis, Fluorescence Properties, and Antiproliferative Potential of Several 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Derivatives" Molecules 20, no. 10: 18565-18584. https://doi.org/10.3390/molecules201018565
APA StyleFu, X.-B., Wang, X.-F., Chen, J.-N., Wu, D.-W., Li, T., Shen, X.-C., & Qin, J.-K. (2015). Synthesis, Fluorescence Properties, and Antiproliferative Potential of Several 3-Oxo-3H-benzo[f]chromene-2-carboxylic Acid Derivatives. Molecules, 20(10), 18565-18584. https://doi.org/10.3390/molecules201018565






