Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans
Abstract
:1. Introduction



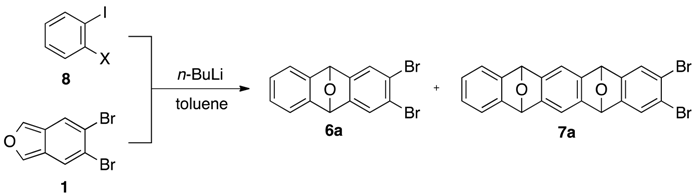
2. Results and Discussion
| Entry | X | Temp. (°C) | Yield of 6a (%) | Yield of 7a (%) 1 |
|---|---|---|---|---|
| 1 | 8a: Br | –78 | 60 | 9 |
| 2 | 8a: Br | –15→25 | 78 | 1 |
| 3 | 8b: Cl | –78 | 51 | 9 |
| 4 | 8b: Cl | –15→25 | 62 | 9 |
| 5 | 8c: F | –78 | 41 | 4 |
| 6 | 8c: F | –15→25 | 44 | 11 |



3. Experimental Section
General Information









4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Hoffmann, R.W. Dehydrobenzene and Cycloalkynes; Academic: New York, NY, USA, 1967. [Google Scholar]
- Kessar, S.V. Nucleophilic coupling with arynes. In Comprehensive Organic Synthesis; Trost, B.M., Ed.; Pergamon: Oxford, UK, 1991; Volume 4, pp. 483–515. [Google Scholar]
- Pellissier, H.; Santelli, M. The use of arynes in organic synthesis. Tetrahedron 2003, 59, 701–730. [Google Scholar]
- Wenk, H.H.; Winkler, M.; Sander, W. One century of aryne chemistry. Angew. Chem. Int. Ed. 2003, 42, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Takaki, K. Aryne insertion reactions into carbon–carbon σ-bonds. Synlett 2012, 23, 1725–1732. [Google Scholar] [CrossRef]
- Bhunia, A.; Yetra, S.R.; Biju, A.T. Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskiy, A.V.; Markina, N.A.; Larock, R.C. Use of benzynes for the synthesis of heterocycles. Org. Biomol. Chem. 2013, 11, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Hamura, T.; Chuda, Y.; Nakatsuji, Y.; Suzuki, K. Catalytic generation of arynes and trapping by nucleophilic addition and iodination. Angew. Chem. Int. Ed. 2012, 51, 3368–3372. [Google Scholar] [CrossRef] [PubMed]
- Haneda, H.; Eda, S.; Aratani, M.; Hamura, T. Dibromoisobenzofuran as a formal equivalent of didehydroisobenzofuran: Reactive platform for expeditious assembly of polycycles. Org. Lett. 2014, 16, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, W. Benzo[c]furans. Adv. Heterocycl. Chem. 1980, 26, 135–241. [Google Scholar]
- Friedrichsen, W. Recent advances in the chemistry of benzo[c]furans and related compounds. Adv. Heterocycl. Chem. 1999, 73, 1–96. [Google Scholar]
- Binger, P.; Wedemann, P.; Goddard, R.; Brinker, U.H. Cyclopropene: A new simple synthesis and Diels-Alder reactions with cyclopentadiene and 1,3-diphenylisobenzofuran. J. Org. Chem. 1996, 61, 6462–6464. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R. Progress in the chemistry of isobenzofurans: Applications to the synthesis of natural products and polyaromatic hydrocarbons. Tetrahedron 1988, 44, 2093–2135. [Google Scholar] [CrossRef]
- Wong, H.N.C. Synthesis of novel benzenoid molecules by low-valent-titanium deoxygenation. Acc. Chem. Res. 1989, 22, 145–152. [Google Scholar] [CrossRef]
- Man, Y.-M.; Mak, T.C.W.; Wong, H.N.C. Synthesis of benzo-fused tetraphenylenes and crystal structure of a 4:1 clathrate inclusion compound of dibenzo[b,h]tetraphenylene with p-xylene. J. Org. Chem. 1990, 55, 3214–3221. [Google Scholar] [CrossRef]
- Chan, S.-H.; Yick, C.-Y.; Wong, H.N.C. 5,6-Bis(trimethylsilyl)benzo[c]furan: An isolable versatile building block for linear polycyclic aromatic compounds. Tetrahedron 2002, 58, 9413–9422. [Google Scholar] [CrossRef]
- Rainbolt, J.E.; Miller, G.P. 4,7-Diphenylisobenzofuran: A useful intermediate for the construction of phenyl-substituted acenes. J. Org. Chem. 2007, 72, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Warrener, R.N. The isolation of isobenzofuran, a stable but highly reactive molecule. J. Am. Chem. Soc. 1971, 93, 2346–2348. [Google Scholar] [CrossRef]
- Warrener, R.N.; Shang, M.; Butler, D.N. A new stabilised form of isobenzofuran, rack-mounted on an alicyclophane. Chem. Commun. 2001, 1550–1551. [Google Scholar] [CrossRef]
- Sygula, A.; Sygula, R.; Rabideau, P.W. Isocorannulenofuran: A versatile building block for the synthesis of large buckybowls. Org. Lett. 2006, 8, 5909–5911. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.J.; Chan, W.H.; Lee, A.W.M. Anthracene capped isobenzofuran: A synthon for the preparations of iptycenes and iptycene quinones. Org. Lett. 2011, 13, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Hamura, T.; Nakayama, R. A one-pot preparation of 1,3-diarylisobenzofuran. Chem. Lett. 2013, 42, 1013–1015. [Google Scholar] [CrossRef]
- Asahina, K.; Matsuoka, S.; Nakayama, R.; Hamura, T. An efficient synthetic route to 1,3-bis(arylethynyl)isobenzofuran using alkoxybenzocyclobutenone as a reactive platform. Org. Biomol. Chem. 2014, 12, 9773–9776. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Xiao Y.; Wang, J.-Y.; Pei, J. Iron(III) chloride promoted cyclization: A facile approach to polycyclic aromatics for functional materials. Synlett 2014, 25, 313–323. [Google Scholar]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. Transannular reactions in asymmetric total synthesis. Tetrahedron 2014, 70, 9461–9484. [Google Scholar] [CrossRef]
- Scott, L.T. Polycyclic aromatic hydrocarbon bowls, baskets, balls and tubes: Challenging targets for chemical synthesis. Polycycl. Aromat. Compd. 2010, 30, 247–259. [Google Scholar] [CrossRef]
- Clayden, J. Regioselective synthesis organolithiums by X-Li exchange. Organolithiums: selectivity for synthesis. In Tetrahedron Organic Chemistry Series; Pergamon: Oxford, UK, 2002; Volume 23, pp. 111–147. [Google Scholar]
- Beak, P.; Allen, D.J. Experimental evaluation of transition structure geometry for an aryl bromide-alkyllithium exchange reaction: New information relevant to the reaction mechanism. J. Am. Chem. Soc. 1992, 114, 3420–3425. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M.; Yus, M. Recent synthetic uses of functionalised aromatic and heteroaromatic organolithium reagents prepared by non-deprotonating methods. Tetrahedron 2003, 59, 9255–9303. [Google Scholar] [CrossRef]
- Dabrowski, M.; Kubicka, J.; Lulinski, S.; Serwatowski, J. Halogen-lithium exchange between substituted dihalobenzenes and butyllithium: Application to the regioselective synthesis of functionalized bromobenzaldehydes. Tetrahedron 2005, 61, 6590–6595. [Google Scholar] [CrossRef]
- Eda, S.; Eguchi, F.; Haneda, H.; Hamura, T. A new synthetic route to substituted tetracenes and pentacenes via stereoselective [4+2] cycloadditions of 1,4-dihydro-1,4-epoxynaphthalene and isobenzofuran. Chem. Commun. 2015, 51, 5963–5966. [Google Scholar] [CrossRef] [PubMed]
- Akita, R.; Kawanishi, K.; Hamura, T. Ring selective generation of isobenzofuran for divergent access to polycyclic aromatic compounds. Org. Lett. 2015, 17, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Gilman, H.; Gorsich, R.D. Some reactions of o-halobromobenzenes with n-butyllithium. J. Am. Chem. Soc. 1956, 78, 2217–2222. [Google Scholar] [CrossRef]
- Chen, L.S.; Chen, G.J.; Tamborski, C. The synthesis and reactins of ortho bromophenyllithium. J. Organomet. Chem. 1980, 193, 283–292. [Google Scholar] [CrossRef]
- Caster, K.C.; Keck, C.G.; Walls, R.D. Synthesis of benzonorbornadienes: Regioselective benzyne formation. J. Org. Chem. 2001, 66, 2932–2936. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.W.; Wirtz, M.C.; Bashore, C.G.; Candler, J. Formation of 3-halobenzyne: Solvent effects and cycloaddition adducts. Org. Lett. 2004, 6, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, A.; Ichinari, D.; Yoshida, J. Three-component coupling based on flash chemistry. Carbolithiation of benzyne with functionalized aryllithiums followed by reactions with electrophiles. J. Am. Chem. Soc. 2014, 136, 12245–12248. [Google Scholar] [CrossRef] [PubMed]
- Voss, G.; Gerlach, H. Regioselektiver brom/lithium-austausch bei 2,5-dibromo-1-nitrobenzol. Eine einfache synthese von 4-brom-2-nitrobenzaldehyd und 6,6′-dibromoindigo. Chem. Ber. 1989, 122, 1199–1201. [Google Scholar] [CrossRef]
- Stanetty, P.; Krumpak, B.; Rodler, I.K. Regioselectivity in sequential bromine-lithium exchange reactions of 2,4-dibromo-N,N-dimethylaniline. J. Chem. Res. 1995, 9, 342–343. [Google Scholar]
- For preparation, see Supporting Information
- Hamura, T.; Arisawa, T.; Matsumoto, T.; Suzuki, K. Two-directional annelation: Dual benzyne cycloadditions starting from bis(sulfonyloxy)diiodobenzene. Angew. Chem. Int. Ed. 2006, 45, 6842–6844. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4946. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. The larger acenes: Versatile organic semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, H.F. Electronic structure of higher acenes and polyacene: The perspective developed by theoretical analyses. Pure Appl. Chem. 2010, 82, 905–915. [Google Scholar] [CrossRef]
- Hart, H.; Ok, D. Synthesis of 1,5-diamino-1,5-dihydrobenzo[1,2-d:4,5-d′]bistriazole (DABT) and its use as a 1,4-benzadiyne equivalent. J. Org. Chem. 1986, 51, 979–986. [Google Scholar] [CrossRef]
- Hart, H.; Lai, C.-Y.; Nwokogu, G.; Shamouilian, S.; Teuerstein, A.; Zlotogorski, C. Bisannelation of arenes with bis-aryne equivalents. J. Am. Chem. Soc. 1980, 102, 6649–6651. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Sun, J.-Q.; Wei, X.; Wong, W.-W.; Lee, A.W.M. Generation of synthetic equivalents of benzdiynes from benzobisoxadisiloles. J. Org. Chem. 2004, 69, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.E.; Barrett, A.G.M. 1,4-Difluoro-2,5-dimethoxybenzene as a precursor for iterative double benzyne-furan Diels-Alder reactions. J. Org. Chem. 2005, 70, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.I.; Cracium, L.; Ho, D.M.; Pascal, R.A., Jr. Synthesis of a strained, air-sensitive, polycyclic aromatic hydrocarbon by means of a new 1,4-benzadiyne equivalent. Tetrahedron 2002, 58, 8875–8882. [Google Scholar] [CrossRef]
- Duong, H.M.; Bendikov, M.; Steiger, D.; Zhang, Q.; Sonmez, G.; Yamada, J.; Wudl, F. Efficient synthesis of a novel, twisted and stable, electroluminescent “twistacene”. Org. Lett. 2003, 5, 4433–4436. [Google Scholar] [CrossRef] [PubMed]
- This site selectivity in the bromine-lithium exchange is due to the electron withdrawal of the four bromo atoms in 16, which leads to the selective generation of tribromophenyllithium. Subsequent elimination of lithium bromide from this intermediate produced dibromobenzyne G.
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eda, S.; Hamura, T. Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans. Molecules 2015, 20, 19449-19462. https://doi.org/10.3390/molecules201019449
Eda S, Hamura T. Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans. Molecules. 2015; 20(10):19449-19462. https://doi.org/10.3390/molecules201019449
Chicago/Turabian StyleEda, Shohei, and Toshiyuki Hamura. 2015. "Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans" Molecules 20, no. 10: 19449-19462. https://doi.org/10.3390/molecules201019449