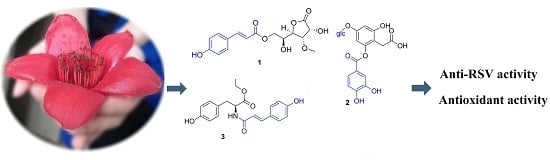
Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities
Abstract
:1. Introduction

2. Results and Discussion
2.1. Identification of Compounds 1–23

| 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Position | δC | δH | Position | δC | δH | Position | δC | δH |
| 1 | 177.4 | - | 1 | 111.9 | - | 1 | 128.9 | - |
| 2 | 73.4 | 4.70 d (4.5) | 2 | 159.0 | - | 2 | 131.4 | 7.03 d (8.4) |
| 3 | 80.2 | 4.16 dd (4.5, 3.6) | 3 | 103.3 | 6.56 d (2.2) | 3 | 116.4 | 6.70 d (8.4) |
| 4 | 79.6 | 4.73 dd (8.1, 3.6) | 4 | 158.6 | - | 4 | 157.6 | - |
| 5 | 74.3 | 5.35 ddd (8.1, 3.6, 3.0) | 5 | 103.7 | 6.47 d (2.2) | 5 | 116.4 | 6.70 d (8.4) |
| 6 | 61.6 | 3.82 m | 6 | 151.9 | - | 6 | 131.4 | 7.03 d (8.4) |
| 1′ | 127.3 | - | 7 | 32.0 | 3.47 s | 7 | 38.1 | 2.93 dd (14.0, 8.0) 3.05 dd (14.0, 6.4) |
| 2′ | 131.4 | 7.47 d (8.4) | 8 | 177.7 | - | 8 | 56.0 | 4.68 dd (8.0, 6.4) |
| 3′ | 117.0 | 6.81 d (8.4) | 1′ | 121.7 | - | 9 | 173.5 | - |
| 4′ | 161.5 | - | 2′ | 118.1 | 7.57 d (2.2) | 10 | 62.4 | 4.12 q (7.1) |
| 5′ | 117.0 | 6.81 d (8.4) | 3′ | 146.5 | - | 11 | 14.5 | 1.19 t (7.1) |
| 6′ | 131.4 | 7.47 d (8.4) | 4′ | 152.6 | - | 1′ | 127.8 | - |
| 7′ | 147.2 | 7.67 d (15.9) | 5′ | 116.1 | 6.87 d (8.4) | 2′ | 130.8 | 7.38 d (8.4) |
| 8′ | 115.2 | 6.38 d (15.9) | 6′ | 124.7 | 7.58 dd (8.4, 2.2) | 3′ | 116.9 | 6.78 d (8.4) |
| 9′ | 168.6 | - | 7′ | 166.6 | 6.45 d (15.9) | 4′ | 160.8 | - |
| 3-OCH3 | 61.1 | 3.60 s | 1′′ | 102.4 | 4.89 (7.6) | 5′ | 116.9 | 6.78 d (8.4) |
| 2′′ | 75.0 | 3.44 | 6′ | 130.8 | 7.38 d (8.4) | |||
| 3′′ | 78.0 | 3.43 | 7′ | 142.7 | 7.42 d (15.9) | |||
| 4′′ | 71.4 | 3.40 | 8′ | 117.9 | 6.45 d (15.9) | |||
| 5′′ | 78.2 | 3.43 | 9′ | 169.1 | - | |||
| 6′′ | 62.6 | 3.88 d (12.0) 3.70 dd (12.0, 3.9) | ||||||
2.2. Antioxidant Activities
| Compounds | DPPH SC50 (μM) a | FRAP Value (μM) b |
|---|---|---|
| 1 | 400.8 ± 25.9 | 28.8 ± 0.9 |
| 2 | 11.3 ± 1.6 | 336.9 ± 17.0 |
| 3 | >500 c | n.d. d |
| 4 | 6.0 ± 0.3 | 139.5 ± 5.0 |
| 5 | 487.1 ± 25.6 | 18.2 ± 0.5 |
| 6 | 10.8 ± 1.2 | 367.1 ± 23.7 |
| 7 | >500 | n.d. |
| 8 | 9.6 ± 0.7 | 379.6 ± 5.2 |
| 9 | >500 | n.d. |
| 10 | 265.4 ± 12.7 | 19.6 ± 0.6 |
| 11 | >500 | n.d. |
| 12 | 14.5 ± 2.3 | 371.3 ± 14.8 |
| 13 | >500 | n.d. |
| 14 | 380.4 ± 22.7 | 35.8 ± 1.0 |
| 15 | 450.8 ± 16.9 | 47.3± 1.2 |
| 16 | >500 | n.d. |
| 17 | >500 | n.d. |
| 18 | >500 | n.d. |
| 19 | 21.3 ± 0.4 | 111.3 ± 1.5 |
| 20 | 27.5 ± 1.3 | 102.5 ± 3.9 |
| 21 | 393.9 ± 11.8 | n.d. |
| 22 | 147.0 ± 8.1 | 12.3 ± 0.6 |
| 23 | 86.1 ± 5.8 | 52.3 ± 1.2 |
| Ascorbic acid | 16.3 ± 0.7 | 417.4 ± 9.8 |
2.3. Anti-RSV Activities
| Compounds | IC50/μM a | CC50/μM b | SI c |
|---|---|---|---|
| 4 | 20.0 ± 0.6 | 258.6 ± 7.9 | 12.9 |
| 10 | 6.3 ± 0.2 | >500.0 | >79.3 |
| 12 | 40.0 ± 0.7 | >500.0 | >12.5 |
| Ribavirin | 10.0 ± 1.3 | 255.9 ± 8.2 | 25.6 |
3. Experimental Section
3.1. General Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Acid Hydrolysis and Sugar Analysis of 2
3.6. Antioxidant Assay
3.7. Cell and Virus
3.8. Anti-RSV Activities
3.9. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium Bromide (MTT) Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An Editorial Committee of Flora of China. Flora of China, 1984 ed.; Science Press: Beijing, China, 1984; Volume 49, p. 106. [Google Scholar]
- Wang, W.P. Preparation and clinical application of dampness granules. Lishizhen Med. Mater. Med. Res. 1999, 10, 336–337. [Google Scholar]
- National pharmacopoeia committee of China. Pharmacopoeia of the People’s Republic of China, 2010 ed.; Chemical Industry Press: Beijing, China, 2010; p. 59. [Google Scholar]
- Yu, Y.G.; He, Q.T.; Yuan, K.; Xiao, X.L.; Li, X.F.; Liu, D.M.; Wu, H. In vitro antioxidant activity of Bombax malabaricum flower extracts. Pharm. Biol. 2011, 49, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Aboutabl, E.A.; Nofal, S.M.; Tokuda, H.; Raslan, M. Phytoconstituents and bioctivity evaluation of Bombax ceiba L. flowers. J. Tradit. Med. 2011, 28, 55–62. [Google Scholar]
- Tundis, R.; Rashed, K.; Said, A.; Menichini, F.; Loizzo, M.R. In vitro cancer cell growth inhibition and antioxidant activity of Bombax ceiba (Bombacaceae) flower extracts. Nat. Prod. Commun. 2014, 9, 691–694. [Google Scholar] [PubMed]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-Ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhu, H.L.; Zhang, S.W.; Yu, Q.; Xuan, L.J. Sesquiterpenoids from Bombax malabaricum. J. Nat. Prod. 2007, 70, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.H.; Zhang, S.W.; Xuan, L.J. Three novel compounds from the flowers of Bombax malabaricum. Helv. Chim. Acta 2008, 91, 136–143. [Google Scholar] [CrossRef]
- Qi, Y.P.; Guo, X.M.; Xia, Z.L.; Liu, J.Y. Studies on flavonoids from the roots of Gossampinus malabaria. Chin. Tradit. Herb. Drugs 2006, 37, 1786–1788. [Google Scholar]
- Turner, A.; Chen, S.N.; Nikolic, D.; Breemen, R.V.; Farnsworth, N.R.; Pauli, G.F. Coumaroyl iridoids and a depside from Cranberry (Vaccinium macrocarpon). J. Nat Prod. 2007, 70, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.Y.; Liu, C.Y.; Cheng, J.Y. Structure and optical activity of natural amino-acid. J. Beijing Inst. Petro-Chem. Technol. 1996, 4, 44–53. [Google Scholar]
- Schut, J.; Bolikal, D.; Khan, I.J.; Pesnell, A.; Rege, A.; Rojas, R.; Sheihet, L.; Murthy, N.S.; Kohn, J. Glass transition temperature prediction of polymers through the mass-per-flexible-bond principle. Polymer 2007, 48, 6115–6124. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Nishiya, K.; Kajikawa, I.; Watanabe, Y.; Suzuki, N.; Takeya, K.; Itokawa, H. Chemical studies on the constituents of Hyphear tanakae Hosokawa from different host trees. Chem. Pharm. Bull. 1988, 36, 1180–1184. [Google Scholar] [CrossRef]
- Rayyan, S.; Fossen, T.; Nateland, H.S.; Andersen, O.M. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug “crataegi folium cum flore” (hawthorn). Phytochem. Anal. 2005, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Isai, N.; Masuda, T.; Honda, G.; Takaishi, Y.; Ito, M.; Otsuka, H.; Ashurmetov, O.A.; Khodzhimatov, O.K. Phlomisflavosides A and B, new flavonol bisglycosides from Phlomis spinidens. Chem. Pharm. Bull. 2001, 49, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, S.; Sakushima, A.; Noro, T.; Fukushima, S. Studies on the Chinese drug Luoshiteng (I). Xanthine oxidase inhibitors from the leaf part of Luoshiteng originating from Trachelospermum jasminoides. Shoyakugaku Zasshi 1987, 41, 116–120. [Google Scholar]
- Beck, M.A.; Häberlein, H. Flavonol glycosides from Eschscholtzia californica. Phytochemistry 1999, 50, 329–332. [Google Scholar] [CrossRef]
- El-Hagrassi, A.M.; Ali, M.M.; Osman, A.F.; Shaaban, M. Phytochemical investigation and biological studies of Bombax malabaricum flowers. Nat. Prod. Res. 2011, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, J.; Zhang, S. Flavonoids from leaves of Heritiera Littoralis D. J. Chin. Pharm. Sci. 2004, 13, 214–216. [Google Scholar]
- Chang, E.J.; Lee, W.J.; Cho, S.H.; Choi, S.W. Proliferative effects of flavan-3-ols and propelargonidins from rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells. Arch. Pharm. Res. 2003, 26, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Hassan, R.A.; Nazif, N.M.; van Miert, S.; Pieters, L.; Hammuda, F.M.; Vlietinck, A.J. Isolation of mangiferin from Bombax malabaricum and structure revision of shamimin. Planta Med. 2003, 69, 1068–1070. [Google Scholar] [PubMed]
- Liu, J.J.; Geng, C.A.; Liu, X.K. A new pyrrolidone derivative from Pistacia chinensis. Chin. Chem. Lett. 2008, 19, 65–67. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, D.; Cui, Z. Ferulates, amurenlactone A and amurenamide A from traditional Chinese medicine cortex Phellodendri Amurensis. J. Asian Nat. Prod. Res. 2008, 10, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Yutaka, O.; Tsutomu, F.; Naomi, H.; Yuichi, D.; Kazuhiko, T. Tsuneyoshi, K. Biotransformation of isoeugenol and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry 1992, 31, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, S.S.; Pei, Y.H. Chemical constituents from leaves of Albizia chinensis. China J. Chin. Mater. Med. 2009, 34, 2063–2066. [Google Scholar]
- Cao, X.; Li, C.J.; Yang, J.Z.; Wei, B.X.; Luo, Y.M.; Zhang, D.M. Study on chemical constituents from leaves of Tripterygium wilfordii. China J. Chin. Mater. Med. 2011, 36, 1028–1031. [Google Scholar]
- Zheng, D.; Zhang, X.Q.; Wang, Y.; Ye, W.C. Chemical constituents of the aerial parts of Blumea riparia. Chin. J. Nat. Med. 2007, 5, 421–424. [Google Scholar]
- Zheng, X.K.; Li, J.; Feng, W.S.; Bi, Y.F.; Ji, C.R. Two new phenylethanoid glycosides from Corallodiscus flabellate. Acta Pharm. Sin. 2003, 38, 268–271. [Google Scholar]
- Abbas, F.A.; Al-massarany, S.M.; Khan, S.; Al-howiriny, T.A.; Mossa, J.S.; Abourashed, E.A. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat. Prod. Res. 2007, 21, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.R.; Zhang, Y.J. Phenolic constituents from the fruits of Amomum tsaoko (Zingiberaceae). Acta Bot. Yunnanica 2009, 31, 284–288. [Google Scholar] [CrossRef]
- OuYang, M.A.; Wang, C.Z.; Wang, S.B. Water-soluble constituents from the leaves of Ilex oblonga. J. Asian Nat. Prod. Res. 2007, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.M.; Chen, Q.; Zhang, L.M.; Chen, S.M. Experimental study on the scavenging of active oxygen species by flavonoids. Acad. J. Sec. Mil. Med. Univ. 1997, 18, 363–365. [Google Scholar]
- Ma, T.X.; Shi, N.; Chen, Q.; Guo, H.J.; Wu, J.H. Comparison on the antioxidant activity of eight components from Rhodiola in vitro. Chin. Pharmacol. Bull. 2012, 28, 1224–1228. [Google Scholar]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; Li, Y.L. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of Citrus reticulata “Chachi”. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; But, P.P.H.; Ooi, V.E.C. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antivir. Res. 2005, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Leung, K.T.; Yao, F.H.; Ooi, L.S.M.; Ooi, V.E.C. Antiviral flavans from the leaves of Pithecellobibium clypearia. J. Nat. Prod. 2006, 69, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–23 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-B.; Wu, P.; Zhang, X.-L.; Xia, C.; Li, G.-Q.; Ye, W.-C.; Wang, G.-C.; Li, Y.-L. Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities. Molecules 2015, 20, 19947-19957. https://doi.org/10.3390/molecules201119660
Zhang Y-B, Wu P, Zhang X-L, Xia C, Li G-Q, Ye W-C, Wang G-C, Li Y-L. Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities. Molecules. 2015; 20(11):19947-19957. https://doi.org/10.3390/molecules201119660
Chicago/Turabian StyleZhang, Yu-Bo, Peng Wu, Xiao-Li Zhang, Chao Xia, Guo-Qiang Li, Wen-Cai Ye, Guo-Cai Wang, and Yao-Lan Li. 2015. "Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities" Molecules 20, no. 11: 19947-19957. https://doi.org/10.3390/molecules201119660
APA StyleZhang, Y.-B., Wu, P., Zhang, X.-L., Xia, C., Li, G.-Q., Ye, W.-C., Wang, G.-C., & Li, Y.-L. (2015). Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities. Molecules, 20(11), 19947-19957. https://doi.org/10.3390/molecules201119660