Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy
Abstract
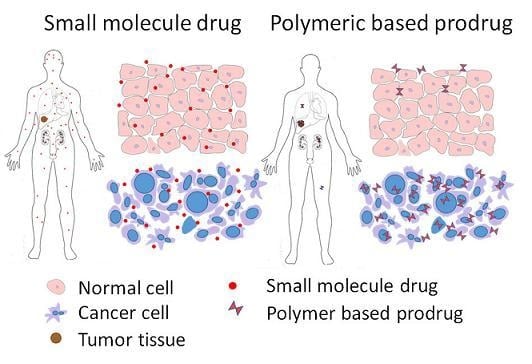
:1. Introduction

2. Classification of Polymers
2.1. Synthetic Polymers
2.1.1. Polyethylene Glycol (PEG)

| Polymer | Application | (co)Polymer | Prodrug | Linker(bond) | Ref. |
|---|---|---|---|---|---|
| PEG | Gene delivery | Polyethylenimine(PEI)-PEG | cDNA of herpes simplex virus thymidine kinase gene (HSVtk) and granulocyte–macrophage colony-stimulating factor (GM-CSF) | N.D. | [21] |
| Tumor targeting | octreotide(Phe)-PEG | Paclitaxel | [22] | ||
| Improved stability and intracellular drug release | methoxy PEG-b-(poly(2-(diisopropylamino) ethyl methacrylate-co-aminopropyl methacrylamide) (PEDPA) | Cis-aconityl-doxorubicin | [23] | ||
| Tumor targeting | PEG | Fusion toxin consisting of the anti-EpCAM DARPin Ec1 and a domain I-deleted variant of ETA (ETA″) | rhinovirus 3C model protease—cleavage linker | [24] | |
| Drug delivery | 3,3′-dithiodipropionic acid functionalized PEG-b-poly(l-lysine) (mPEG-b-P(LL-DTPA)) | Paclitaxel | Disulfide | [25] | |
| Multidrug resistance | D-α-tocopherol PEG succinate (TPGS) | Paclitaxel | Disulfide | [26] | |
| Improved the therapeutic efficacy | β-CD, PEG | Doxorubicin | hydrazone | [27] | |
| Tumor targeting | PEG | Paclitaxel | [28] | ||
| Multidrug resistance | PEG-poly(d,l-lactide) | 4-(N)-stearoyl Gemcitabine | [29] | ||
| Theranosis | PEG-polylactic acid (PEG-PLA) l | Dicyanomethylene-4H-pyran-S-CPT | Disulfide | [30] | |
| Tumor targeting | PEG monomethyl ether (mPEG) | Artesunate | Ester | [31] | |
| Tumor targeting | PEG monomethyl ether | Camptothecin | Disulfide | [32] | |
| Tumor targeting | PEG | Camptothecin | Disulfide | [33] | |
| Tumor targeting | PEG2000 | Paclitaxel | MMP2-cleavable linker | [34] | |
| Nanogel | PLGA-PEG-PLGA | PEGylated Taxol | [35] | ||
| Drug delivery | mPEG-b-P(ATMC-co-DTC) | Doxorubicin | Hydrazone | [36] | |
| HPMA | improving anticancer therapy | (mPEG5000-b-p(HPMAmLac2-r-AzEMA) | Doxorubicin-glucuronide prodrug (DOX-propGA3) | Glucuronide (β-glucuronidase cleavable linker) | [37] |
| Prevent metastasis | HPMA copolymer | E-selectin binding peptide (Esbp)-doxorubicinor (KLAKLAK)2 | [38] | ||
| Drug delivery | HPMA copolymer | H1-S6A, F8A peptide | GFLG (Cathepsin cleavage linker) | [39] | |
| Tumor targeting | HPMA copolymer | Doxorubicin | GFLG and MMP cleavable linker | [40] | |
| Improved the therapeutic efficacy | HPMA copolymer | Doxorubicin, 5-FU | Hydrazone, GFLG | [41] | |
| Theranosis | Star polymer: poly(amido amine) (PAMAM) dendrimers and HPMA | Doxorubicin or TAMRA fluorescent dye | Hydrazone | [42] | |
| Improved Bioavailability | Star polymer: poly(amido amine) (PAMAM) dendrimers and HPMA | Pirarubicin | Hydrazone | [43,44] | |
| Drug delivery | HPMA copolymer | Iodine-125 | Hydrazone | [45] | |
| Drug delivery | HPMA copolymer | Paclitaxel, Gemcitabine | GFLG | [46] | |
| Drug delivery | Starch + HPMA copolymer | Camptothecin | [47] | ||
| Gene delivery | galactosylated 2-hydroxypropylmethacrylamide-s-3-guanidinopropyl methacrylamide (HPMAs–GPMA) | shRNA | [48] | ||
| Drug delivery | HPMA | Indium-111, Yttrium-90 | [49] | ||
| Improved the therapeutic efficacy | multiblock poly HPMA | Gemcitabine, Paclitaxel and Doxorubicin | GFLG | [50] | |
| Theranosis | HPMA copolymer | Zinc protoporphyrin | [16,51] | ||
| SMA | Photodynamic therapy | SMA | Zinc protoporphyrin | Amide | [52] |
| PLGA | Improved Bioavailability | PLGA | Gemcitabine | Amide | [53] |
| PGG | Improved Bioavailability | PGG | Paclitaxel | Glutamic acid | [54,55] |
2.1.2. The N-(2-hydroxypropyl) Methacrylamide (HPMA) Copolymer

2.1.3. Poly (Styrene-Co-Maleic Acid/Anhydride) (SMA)

2.1.4. The Polyglutamic Acid Polymer

2.1.5. Poly (Lactic-Co-Glycolic Acid) (PLGA)

2.2. Natural Polymers
2.2.1. Chitosan

2.2.2. Dextran

| Polymer | Name | Drug | Status | Ref. |
|---|---|---|---|---|
| Dextran | OsteoDex | Alendronate | Phase I | [21,78,79] |
| Somadex | Somatostatin | Phase I | [78] | |
| PEG | NK105 | Paclitaxel | Phase III | [21] |
| NK102 | SN-38 | Phase II | [80,81] | |
| NC-6004 | Cisplatin | Phase III | [21] | |
| NC-4016 | Dachplatin | Phase I | [21] | |
| NC-6300 | Doxorubicin | Phase I | [21] | |
| poly-l-glutamate | paclitaxel poliglumex, CT-2103 | Paclitaxel | Phase III | [82,83] |
| Cyclodextrin-PEG copolymer | CRLX101 | Camptothecin | Phase II | [84] |
2.2.3. Pullulan

Genetically Engineered Polypeptides
2.2.4. Elastin-Like Polypeptide (ELP)

2.2.5. Silk-Elastin Like Polypeptides
3. Polymer Drug Conjugates in Clinical Trials
3.1. Dextran Conjugates
3.2. HPMA Conjugates
3.3. PEG Conjugates
3.3.1. EZN-2208/BEL-0222
3.3.2. NC-6300
4. Conclusions and Future Direction
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.N. Solid dispersions, part II: New strategies in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1663–1680. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.; Sandhu, H.; Singhal, D.; Malick, W.; Shah, N.; Serpil Kislalioglu, M. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr. Drug Deliv. 2012, 9, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R. Nanoparticles: A personal experience for formulating poorly water soluble drugs. J. Control. Release 2010, 141, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J. Pharm. Pharm. Sci. 2008, 11, 32–58. [Google Scholar] [PubMed]
- Venkatesh, S.; Li, J.; Xu, Y.; Vishnuvajjala, R.; Anderson, B.D. Intrinsic solubility estimation and pH-solubility behavior of cosalane (NSC 658586), an extremely hydrophobic diprotic acid. Pharm. Res. 1996, 13, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.D.; Flora, K.; Goldspiel, B.; Wilson, J.; Arbuck, S.; Finley, R. Taxol: A history of pharmaceutical development and current pharmaceutical concerns. J. Natl. Cancer Inst. Monogr. 1992, 141–147. [Google Scholar]
- Arbuck, S.G.; Christian, M.; Fisherman, J.; Cazenave, L.; Sarosy, G.; Suffness, M.; Adams, J.; Canetta, R.; Cole, K.; Friedman, M. Clinical development of Taxol. J. Natl. Cancer Inst. Monogr. 1992, 11–24. [Google Scholar]
- Dorr, R.T. Pharmacology and toxicology of Cremophor EL diluent. Ann. Pharmacother. 1994, 28, S11–S14. [Google Scholar] [PubMed]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Giang, I.; Boland, E.L.; Poon, G.M. Prodrug applications for targeted cancer therapy. AAPS J. 2014, 16, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Johnson, R.P.; Heo, M.S.; Moon, B.K.; Byeon, S.J.; Kim, I. Polymer-Block-Polypeptides and Polymer-Conjugated Hybrid Materials as Stimuli-Responsive Nanocarriers for Biomedical Applications. J. Biomed. Nanotechnol. 2015, 11, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Salehi Moghaddam, Z.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli-responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.S.; Raucher, D. Elastin-like polypeptide for improved drug delivery for anticancer therapy: Preclinical studies and future applications. Expert Opin. Drug Deliv. 2015, 12, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sima, M.; Yang, J.; Kopeček, J. Synthesis of Long-Circulating, Backbone Degradable HPMA Copolymer–Doxorubicin Conjugates and Evaluation of Molecular-Weight-Dependent Antitumor Efficacy. Macromol. Biosci. 2013, 13, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Kopeček, J. Soluble synthetic polymers as potential drug carriers. In Polymers in Medicine; Springer: Berlin, Heidelberg, Germany, 1984; Volume 57, pp. 51–101. [Google Scholar]
- Jang, H.-J.; Shin, C.Y.; Kim, K.-B. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Du, H.L.; Zhang, H.Q.; Zhai, Y.J.; Zhai, G.X. Polymer-drug conjugates: Present state of play and future perspectives. Drug Discov. Today 2013, 18, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Rohini, N.A.; Joseph, A.; Mukerji, A. Polymeric prodrugs: Recent achievements and general strategies. J. Antivir. Antiretrovir. 2013. [Google Scholar] [CrossRef]
- Alekseenko, I.V.; Snezhkov, E.V.; Chernov, I.P.; Pleshkan, V.V.; Potapov, V.K.; Sass, A.V.; Monastyrskaya, G.S.; Kopantzev, E.P.; Vinogradova, T.V.; Khramtsov, Y.V.; et al. Therapeutic properties of a vector carrying the HSV thymidine kinase and GM-CSF genes and delivered as a complex with a cationic copolymer. J. Transl. Med. 2015, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhu, Q.; Wu, Q.; Yin, T.; Wang, L.; Yin, L.; Zhou, J. Somatostatin receptor-mediated specific delivery of paclitaxel prodrugs for efficient cancer therapy. J. Pharm. Sci. 2015, 104, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, W.; Zhou, J.; Zhao, F.; Zhang, Y.; Liu, J.; Liu, J.; Dong, A.; Kong, D.; Zhang, J. Amphiphilic polyelectrolyte/prodrug nanoparticles constructed by synergetic electrostatic and hydrophobic interactions with cooperative pH-sensitivity for controlled doxorubicin delivery. ACS Appl. Mater. Interfaces 2015, 7, 6340–6350. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Zimmermann, M.; Simon, M.; Zangemeister-Wittke, U.; Pluckthun, A. Novel prodrug-like fusion toxin with protease-sensitive bioorthogonal PEGylation for tumor targeting. Bioconjug. Chem. 2014, 25, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Tang, Z.; Zhang, D.; Song, W.; Li, M.; Lin, J.; Liu, H.; Chen, X. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J. Control. Release 2014, 194, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Guo, Y.; Zhuang, X.; Li, D.; Cheng, B.; Tan, S.; Zhang, Z. D-alpha-tocopherol polyethylene glycol succinate-based redox-sensitive paclitaxel prodrug for overcoming multidrug resistance in cancer cells. Mol. Pharm. 2014, 11, 3196–3209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Chen, Y.; Liu, X.; Jin, Q.; Ji, J. pH and hydrogen peroxide dual responsive supramolecular prodrug system for controlled release of bioactive molecules. Colloids Surf. B Biointerfaces 2014, 121, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.P.; Park, J.K.; Son, D.H.; Kim, T.H.; Park, S.J.; Park, S.C.; Choi, C.; Jang, M.K.; Nah, J.W. Evaluation of polyethylene glycol-conjugated novel polymeric anti-tumor drug for cancer therapy. Colloids Surf. B Biointerfaces 2014, 120, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Daman, Z.; Ostad, S.; Amini, M.; Gilani, K. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: A comparison with its self-assembled nanoparticles. Int. J. Pharm. 2014, 468, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, X.; Guo, Z.; Tang, J.; Shen, Y.; James, T.D.; Tian, H.; Zhu, W. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Du, F.; Huang, J.; Lu, W.; Yu, J.; Liu, S.; Muir, B. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Colloids Surf. B 2014, 115, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, D.; Xu, S.; Liu, X.; Zhang, X.; Zhang, H. Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chem. Asian J. 2014, 9, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.A.; Kularatne, S.A.; Hakenjos, J.; Carron, J.D.; Henne, K.L. Synthesis and activity of a folate targeted monodisperse PEG camptothecin conjugate. Bioorg. Med. Chem. Lett. 2013, 23, 5810–5813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, H.; Zhu, M.; Ding, D.; Li, D.; Yin, Z.; Wang, L.; Yang, Z. Janus nanogels of PEGylated Taxol and PLGA-PEG-PLGA copolymer for cancer therapy. Nanoscale 2013, 5, 9902–9907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, Y.M.; Lv, Y.; Cheng, Y.J.; He, F.; Zhuo, R.X. Amphiphilic polycarbonate conjugates of doxorubicin with pH-sensitive hydrazone linker for controlled release. Colloids Surf. B 2013, 111, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Morita, K.; Rijcken, C.J.; Aben, R.W.; Lammers, T.; Scheeren, H.W.; van Nostrum, C.F.; Storm, G.; Hennink, W.E. Synthesis and characterization of biodegradable and thermosensitive polymeric micelles with covalently bound doxorubicin-glucuronide prodrug via click chemistry. Bioconjug. Chem. 2011, 22, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Shamay, Y.; Raviv, L.; Golan, M.; Voronov, E.; Apte, R.N.; David, A. Inhibition of primary and metastatic tumors in mice by E-selectin-targeted polymer-drug conjugates. J. Control. Release 2015, 217, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, L.; Zhu, X.; Guan, S.; Yang, Q.; Zhou, Z.; Zhang, Z.; Huang, Y. A smart polymeric platform for multistage nucleus-targeted anticancer drug delivery. Biomaterials 2015, 65, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.H.; Kopecek, J. Enhancing Accumulation and Penetration of HPMA Copolymer-Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. J. Am. Chem. Soc. 2015, 137, 6726–6729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, Y.; Li, L.; Sun, W.; Zhu, X.; Huang, Y. Polymeric nanomedicine for tumor-targeted combination therapy to elicit synergistic genotoxicity against prostate cancer. ACS Appl. Mater. Interfaces 2015, 7, 6661–6673. [Google Scholar] [CrossRef] [PubMed]
- Chytil, P.; Koziolova, E.; Janouskova, O.; Kostka, L.; Ulbrich, K.; Etrych, T. Synthesis and Properties of Star HPMA Copolymer Nanocarriers Synthesised by RAFT Polymerisation Designed for Selective Anticancer Drug Delivery and Imaging. Macromol. Biosci. 2015, 15, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Koziolova, E.; Etrych, T.; Chytil, P.; Fang, J.; Ulbrich, K.; Maeda, H. Comparison between linear and star-like HPMA conjugated pirarubicin (THP) in pharmacokinetics and antitumor activity in tumor bearing mice. Eur. J. Pharm. Biopharm. 2015, 90, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Etrych, T.; Chytil, P.; Ohkubo, M.; Fang, J.; Ulbrich, K.; Maeda, H. Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage. J. Control. Release 2014, 174, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, O.; Kucka, J.; Mattova, J.; Parizek, M.; Studenovsky, M.; Zadinova, M.; Pouckova, P.; Hruby, M. Multistage-targeted pH-responsive polymer conjugate of Auger electron emitter: optimized design and in vivo activity. Eur. J. Pharm. Sci. 2014, 63, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, J.; Sima, M.; Zhou, Y.; Kopecek, J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc. Natl. Acad. Sci. USA 2014, 111, 12181–12186. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.; Murugan, D.; Rengarajan, A.; Rajiv, S. Anticancer activity of starch/poly[N-(2-hydroxypropyl)methacrylamide]: Biomaterial film to treat skin cancer. Int. J. Biol. Macromol. 2014, 70, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ji, J.; Yang, R.; Zhang, X.; Li, Y.; Pu, Y.; Li, X. Galactosylated 2-hydroxypropyl methacrylamide-s-3-guanidinopropyl methacrylamide copolymer as a small hairpin RNA carrier for inhibiting human telomerase reverse transcriptase expression. J. Gene Med. 2014, 16, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Buckway, B.; Frazier, N.; Gormley, A.J.; Ray, A.; Ghandehari, H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer-90Y conjugates in treatment of prostate tumors. Nucl. Med. Biol. 2014, 41, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Yang, J.; Ray, A.; Cheney, D.L.; Ghandehari, H.; Kopecek, J. Biodegradable multiblock poly(N-2-hydroxypropyl)methacrylamide gemcitabine and paclitaxel conjugates for ovarian cancer cell combination treatment. Int. J. Pharm. 2013, 454, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Liao, L.; Hitaka, Y.; Tsukigawa, K.; Subr, V.; Fang, J.; Ulbrich, K.; Maeda, H. Micelles of zinc protoporphyrin conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer for imaging and light-induced antitumor effects in vivo. J. Control. Release 2013, 165, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kayat, J.; Mehra, N.K.; Gajbhiye, V.; Jain, N.K. Drug targeting to arthritic region via folic acid appended surface-engineered multi-walled carbon nanotubes. J. Drug Target. 2015, 1–10. [Google Scholar]
- Khare, V.; Kour, S.; Alam, N.; Dubey, R.D.; Saneja, A.; Koul, M.; Gupta, A.P.; Singh, D.; Singh, S.K.; Saxena, A.K.; et al. Synthesis, characterization and mechanistic-insight into the anti-proliferative potential of PLGA-gemcitabine conjugate. Int. J. Pharm. 2014, 470, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Van, S.; Das, S.K.; Wang, X.; Feng, Z.; Jin, Y.; Hou, Z.; Chen, F.; Pham, A.; Jiang, N.; Howell, S.B. Synthesis, characterization, and biological evaluation of poly (l-γ-glutamyl-glutamine)-paclitaxel nanoconjugate. Int.J. Nanomed. 2010, 5, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, X.; Jiang, X.; Liu, Y.; Ying, W.; Wang, H.; Bai, H.; Taylor, W.D.; Wang, Y.; Clamme, J.-P. Effect of molecular weight of PGG–paclitaxel conjugates on in vitro and in vivo efficacy. J. Control. Release 2012, 161, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, G.; Shi, C.; Guo, D.; Wang, X.; Luo, J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials 2015, 37, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Song, H.; Yang, Q.; Cai, H.; Qi, R.; Yan, L.; Liu, S.; Zheng, Y.; Huang, Y.; Liu, T. A prodrug strategy to deliver cisplatin (IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials 2012, 33, 6507–6519. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Vicent, M.J. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv. Drug Deliv. Rev. 2010, 62, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Greish, K.; Bauer, H.; Maeda, H.; Ghandehari, H. Synthesis and evaluation of poly(styrene-co-maleic acid) micellar nanocarriers for the delivery of tanespimycin. Int. J. Pharm. 2011, 420, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Kamada, H.; Kaneda, Y.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Tsutsumi, Y.; Maeda, M.; Kawasaki, K.; Nomizu, M.; et al. YBioconjugation of laminin peptide YIGSR with poly(styrene co-maleic acid) increases its antimetastatic effect on lung metastasis of B16-BL6 melanoma cells. Biochem. Biophys. Res. Commun. 1999, 255, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185. [Google Scholar] [CrossRef]
- Maeda, H.; Ueda, M.; Morinaga, T.; Matsumoto, T. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: Pronounced improvements in pharmacological properties. J. Med. Chem. 1985, 28, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Tsukigawa, K.; Liao, L.; Yin, H.; Eguchi, K.; Maeda, H. Styrene-maleic acid-copolymer conjugated zinc protoporphyrin as a candidate drug for tumor-targeted therapy and imaging. J. Drug Target. 2015, 1–9. [Google Scholar]
- Bae, H.H.; Cho, M.Y.; Hong, J.H.; Poo, H.; Sung, M.H.; Lim, Y.T. Bio-derived poly(gamma-glutamic acid) nanogels as controlled anticancer drug delivery carriers. J. Microbiol. Biotechnol. 2012, 22, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Heo, M.B.; Lim, Y.T. Poly (gamma-glutamic acid) based combination of water-insoluble paclitaxel and TLR7 agonist for chemo-immunotherapy. Biomaterials 2014, 35, 7992–8001. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Hasanzadeh, F.; Sadeghi, H.; Mostafavi, A.; Minaiyan, M.; Rostami, M.; Davies, N. Development and in vitro/in vivo evaluation of a novel targeted polymeric micelle for delivery of paclitaxel. Int. J. Biol. Macromol. 2015, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Gorska, A.; Swiatkowska, A.; Dutkiewicz, M.; Ciesiolka, J. Modulation of p53 expression using antisense oligonucleotides complementary to the 5′-terminal region of p53 mRNA in vitro and in the living cells. PLoS ONE 2013, 8, e78863. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Shigdar, S.; Qiao, G.; Wang, T.; Kouzani, A.Z.; Zhou, S.F.; Kong, L.; Li, Y.; Pu, C.; Duan, W. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: The next generation of cancer medicine. Theranostics 2015, 5, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z. In vivo antitumor activity of chitosan nanoparticles. Bioorg. Med. Chem. Lett. 2006, 16, 4243–4245. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Scheeren, L.E.; Macedo, L.B.; Marcolino, A.I.; Pilar Vinardell, M.; Mitjans, M.; Rosa Infante, M.; Farooqi, A.A.; Rolim, C.M. Inclusion of a pH-responsive amino acid-based amphiphile in methotrexate-loaded chitosan nanoparticles as a delivery strategy in cancer therapy. Amino Acids 2015, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, T.; Mitra, S.; Kumar Singh, A.; Kumar Sharma, R.; Maitra, A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Rubino, F.M. Separation methods for methotrexate, its structural analogues and metabolites. J. Chromatogr. B 2001, 764, 217–254. [Google Scholar] [CrossRef]
- Nam, J.P.; Lee, K.J.; Choi, J.W.; Yun, C.O.; Nah, J.W. Targeting delivery of tocopherol and doxorubicin grafted-chitosan polymeric micelles for cancer therapy: In vitro and in vivo evaluation. Colloids Surf. B 2015, 133, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.Y.; Rauth, A.M.; Ronaldson, P.T.; Bendayan, R.; Wu, X.Y. In vitro toxicity to breast cancer cells of microsphere-delivered mitomycin C and its combination with doxorubicin. Eur. J. Pharm. Biopharm. 2006, 62, 321–331. [Google Scholar] [CrossRef] [PubMed]
- DexTch Medical. Available online: http://dextechmedical.com (accessed on 30 November 2015).
- ClinicalTrials.gov. Available online: http://clinicaltrials.gov (NCT01595087) (accessed on 30 November 2015).
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. The drug discovery by nanomedicine and its clinical experience. Jpn. J. Clin. Oncol. 2014, 44, 515–525. [Google Scholar] [CrossRef] [PubMed]
- CTI BioPharma. Available online: http://www.ctibiopharma.com (accessed on 30 November 2015).
- Langer, C.J.; O′Byrne, K.J.; Socinski, M.A.; Mikhailov, S.M.; Lesniewski-Kmak, K.; Smakal, M.; Ciuleanu, T.E.; Orlov, S.V.; Dediu, M.; Heigener, D.; et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Cerlulean. Available online: http://www,ceruleanrx.com (accessed on 30 November 2015).
- Lee, K.D.; Choi, S.H.; Kim da, H.; Lee, H.Y.; Choi, K.C. Self-organized nanoparticles based on chitosan-folic acid and dextran succinate-doxorubicin conjugates for drug targeting. Arch. Pharm. Res. 2014, 37, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Ponrasu, T.; Raja, M.D.; Subamekala, M.K.; Suguna, L. Green synthesis of pullulan stabilized gold nanoparticles for cancer targeted drug delivery. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Scomparin, A.; Salmaso, S.; Bersani, S.; Satchi-Fainaro, R.; Caliceti, P. Novel folated and non-folated pullulan bioconjugates for anticancer drug delivery. Eur. J. Pharm. Sci. 2011, 42, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Malet-Martino, M.; Martino, R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): A review. Oncologist 2002, 7, 288–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, F.; Yi, J.; Gu, C.; Fan, L.; Qiao, Y.; Tao, Y.; Cheng, C.; Wu, H. Folate-decorated maleilated pullulan-doxorubicin conjugate for active tumor-targeted drug delivery. Eur. J. Pharm. Sci. 2011, 42, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Moktan, S.; Ryppa, C.; Kratz, F.; Raucher, D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Investig. New Drugs 2012, 30, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Mikecin, A.M.; Walker, L.R.; Kuna, M.; Raucher, D. Thermally targeted p21 peptide enhances bortezomib cytotoxicity in androgen-independent prostate cancer cell lines. Anticancer Drugs 2014, 25, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Moktan, S.; Perkins, E.; Kratz, F.; Raucher, D. Thermal targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo. Mol. Cancer Ther. 2012, 11, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Massodi, I.; Bidwell, G.L., 3rd; Davis, A.; Tausend, A.; Credit, K.; Flessner, M.; Raucher, D. Inhibition of ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP. Clin. Exp. Metastasis 2009, 26, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, G.L., 3rd; Perkins, E.; Hughes, J.; Khan, M.; James, J.R.; Raucher, D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS ONE 2013, 8, e55104. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, G.L., 3rd; George, E.M. Maternally sequestered therapeutic polypeptides—A new approach for the management of preeclampsia. Front. Pharmacol. 2014, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Amruthwar, S.S.; Janorkar, A.V. In vitro evaluation of elastin-like polypeptide-collagen composite scaffold for bone tissue engineering. Dent. Mater. 2013, 29, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, G.L., 3rd; Davis, A.N.; Fokt, I.; Priebe, W.; Raucher, D. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Investig. New Drugs 2007, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Andrew MacKay, J.; Chen, M.; McDaniel, J.R.; Liu, W.; Simnick, A.J.; Chilkoti, A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 2009, 8, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.; Nunalee, M.L.; Lim, D.W.; Simnick, A.J.; Chilkoti, A. Peptide-based Biopolymers in Biomedicine and Biotechnology. Mater. Sci. Eng. R Rep. 2008, 62, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Wang, M.; Lin, Y.; Xu, Q.; Kaplan, D.L. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules 2014, 15, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Ferry, D.R.; Kerr, D.J.; Rea, D.; Whitlock, M.; Poyner, R.; Boivin, C.; Hesslewood, S.; Twelves, C.; Blackie, R.; et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int. J. Oncol. 2009, 34, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Papadopoulos, K.P.; Tolcher, A.W.; Beeram, M.; Urien, S.; Schaaf, L.J.; Tahiri, S.; Bekaii-Saab, T.; Lokiec, F.M.; Rezai, K.; et al. Phase I dose-escalation study of EZN-2208 (PEG-SN38), a novel conjugate of poly(ethylene) glycol and SN38, administered weekly in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 71, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- BelRose Pharma. Available online: http://belrosepharma.com (accessed on 30 November 2015).
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules 2015, 20, 21750-21769. https://doi.org/10.3390/molecules201219804
Dragojevic S, Ryu JS, Raucher D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules. 2015; 20(12):21750-21769. https://doi.org/10.3390/molecules201219804
Chicago/Turabian StyleDragojevic, Sonja, Jung Su Ryu, and Drazen Raucher. 2015. "Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy" Molecules 20, no. 12: 21750-21769. https://doi.org/10.3390/molecules201219804
APA StyleDragojevic, S., Ryu, J. S., & Raucher, D. (2015). Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules, 20(12), 21750-21769. https://doi.org/10.3390/molecules201219804