New 1H-Benzo[f]indazole-4,9-diones Conjugated with C-Protected Amino Acids and Other Derivatives: Synthesis and in Vitro Antiproliferative Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry




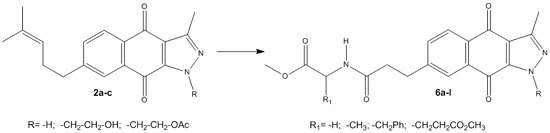
- -
- In some cases, their IR spectra show two carbonyl-quinone absorptions at approximately 1680 and 1670 cm−1, but the latter absorption is primarily observed.
- -
- In the 1H spectra, the singlet of the C-10 methyl group appears at approximately 2.60 to 2.80 ppm, the coupled methylene groups of C-11 and C-12 carbons show triplets or multiplets between 2.50 and 3.00 ppm (J = 7.3–8.0 Hz), and the coupled aromatic hydrogen of carbon C-5, C-6 and C-8 are observed as doublets of doublets and two doublets at 7.70 to 8.10 ppm (J = 7.6 and 1.6 Hz).
- -
- The 13C-NMR spectra contain signals for carbonyl-quinone C-4 and C-9 carbon atoms at 170 to 180 ppm.
2.2. Biological Assay
| Series-I | Series-II | Series-III |
|---|---|---|
| IC50 (CI 95%) μM | IC50 (CI 95%) μM | IC50 (CI 95%) μM |
| p | p | p |
| 2a | 2b | 2a |
| 60.3 (18.9–192.4) | 25.5 (9.4–69.1) | 33.0 (8.0–136.5) |
| C: NA, R: NA | C: NA, R: NS | C: NA, R: NS |
| 3a | 3b | 3c |
| 313.3 (110.4–889.3) | 401.8 (166.8–967.8) | 324.3 (132.9–791.3) |
| C: ***, R: NA | C: ****, R: NS | C: ****, R: NS |
| 4a | 4b | 4c |
| 99.5 (50.1–197.7) | 63.0 (22.4–176.9) | 60.5 (29.8–123.1) |
| C: ***, R: NA | C: NS, R: NS | C: NS, R: NS |
| 5a | 5b | 5c |
| 326.6 (167.9–635.4) | 337.3 (192.3–591.5) | 162.6 (70.6–374.2) |
| C: ***, R: NA | C: ****, R: NS | C **, R: NS |
| 6a | 6e | 6i |
| 230.7 (82.9–642.2) | 310.5 (132.5–727.4) | 208.0 (92.5–467.6) |
| C: **, R: NA | C: ****, R: NS | C: ***, R: NS |
| 6b | 6f | 6j |
| 114.8 (57.7–228.6) | 43.5 (15.9–118.4) | 54.1 (13.6–215.1) |
| C: NS, R: NA | C: NS, R: * | C: NS, R: NS |
| 6c | 6g | 6k |
| 126.8 (41.3–389.4) | 37.6 (11.5–123.4) | 34.9 (16.7–72.9) |
| C: NS, R: NA | C: NS, R: * | C: NS, R: ** |
| 6d | 6h | 6l |
| 111.7 (35.3–353.1) | 52.8 (16.8–166.7) | 109.6 (32.5–370.4) |
| C: NS, R: NA | C: NS, R: NS | C: NS, R: NS |
| Series-I | Series-II | Series-III |
|---|---|---|
| IC50 (CI 95%) μM | IC50 (CI 95%) μM | IC50 (CI 95%) μM |
| p | p | p |
| 2a | 2b | 2c |
| 63.2 (24.8–161.5) | 27.5 (71.1–106.8) | 29.4 (14.1–61.3) |
| C: NA, R: NA | C: NA, R: NS | C: NA, R: NS |
| 3a | 3b | 3c |
| 432.5 (167.8–1115.1) | 415.9 (202.0–856.2) | 389.9 (222.2–684.4) |
| C: ****, R: NA | C: ****, R: NS | C: ****, R: NS |
| 4a | 4b | 4c |
| 123.6 (39.6–385.7) | 43.4 (18.6–101.1) | 33.0 (10.8–100.7) |
| C: NS, R: NA | C: NS, R: * | C: NS, R: ** |
| 5a | 5b | 5c |
| 372.4 (185.1–749.2) | 335.0 (186.7–601.1) | 244.9 (95.0–631.0) |
| C: ***, R: NA | C: ****, R: NS | C: ****, R: NS |
| 6a | 6e | 6i |
| 413.0 (138.2–1234.7) | 291.1 (155.1–546.2) | 255.3 (115.8–562.8) |
| C: ****, R: NA | C: ****, R: NS | C: ****, R: NS |
| 6b | 6f | 6j |
| 94.2 (44.8–197.9) | 62.7 (17.0–231.2) | 52.6 (24.2–114.2) |
| C: NS, R: NA | C: NS, R: NS | C: NS, R: NS |
| 6c | 6g | 6k |
| 154.9 (59.2–405.2) | 39.0 (11.6–131.1) | 35.4 (8.4–149.5) |
| C: NS, R: NA | C: NS, R: ** | C: NS, R: *** |
| 6d | 6h | 6l |
| 143.9 (60.3–343.5) | 87.9 (45.0–171.7) | 99.8 (52.2–190.6) |
| C: NS, R: NA | C: NS, R: NS | C: NS, R: NS |
3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for the Preparation of 7-[2-(3,3-Dimethyloxiranyl)-ethyl]-3-methyl-1H-benzo[f]indazole-4,9-diones 3a–c
3.1.3. General Procedure for the Preparation of N-Substituted 3-(3-Methyl-4,9-dioxo-4,9-dihydro-1H-benzo[f]indazol-7-yl)-propanal 4a–c
3.1.4. General Procedure for the Preparation of N-Substituted 3-(3-Methyl-4,9-dioxo-4,9-dihydro-1H-benzo[f]indazol-7-yl)-propanoic Acids 5a–c
3.1.5. General Procedure for the Preparation of [3-(3-Methyl-4,9-dioxo-4,9-dhydro-1H-benzo[f]indazol-7-yl)propanoylamino]-methyl Ester 6a–l
3.2. Computational Details
3.3. Antiproliferative Assay
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foye, W.O. Cancer Chemotherapeutic Agents; ACS: Washington, DC, USA, 1995. [Google Scholar]
- Hertzberg, R.P.; Dervan, P.B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): Reaction conditions and product analyses. Biochemistry 1984, 23, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Suh, M.; Lee, C. Synthesis and cytotoxicity evaluation of 2-amino- and 2-hydroxy-3-ethoxycarbonyl-N-substituted-benzo[f]indole-4,9-dione derivatives. Bioorg. Med. Chem. 2003, 11, 1511–1519. [Google Scholar] [CrossRef]
- Brunmark, A.; Cadenas, E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic. Biol. Med. 1989, 7, 435–477. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact 1991, 80, 1–40. [Google Scholar] [CrossRef]
- Montoya, J.; Varela-Ramírez, A.; Shanmugasundram, M.; Martínez, L.E.; Primm, T.P.; Aguilera, R.J. Tandem screening of toxic compounds on GFP-labeled bacteria and cancer cells in microtiter plates. Biochem. Biophys. Res. Commun. 2005, 335, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Benites, J.; Valderrama, J.A.; Rivera, F.; Rojo, L.; Campos, N.; Pedro, M.; Nascimento, M.S. Studies on quinones. Part 42: Synthesis of furylquinone and hydroquinones with antiproliferative activity against human tumor cell lines. Part 42. Bioorg. Med. Chem. 2008, 16, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Conway, G.A.; Loeffler, L.J.; Hall, I.H. Synthesis and antitumor evaluation of selected 5,6-disubstituted 1(2)H-indazole-4,7-diones. J. Med. Chem. 1983, 26, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, R.; Li, G.; Xue, X.; Sun, C.; Qu, X.; Li, W. Synthesis of indazole based diarylurea derivatives and their antiproliferative activity against tumor cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Buchstaller, H.; Eggenweiler, H.; Sirrenberg, C.; Grädler, U.; Musil, D.; Hoppe, E.; Zimmermann, A.; Schwartz, H.; März, J.; Bomke, J.; et al. Fragment-based discovery of hydroxy-indazole-carboxamides as novel small molecule inhibitors of Hsp90. Bioorg. Med. Chem. Lett. 2012, 22, 4396–4403. [Google Scholar] [CrossRef] [PubMed]
- Shakil, N.A.; Singh, M.K.; Sathiyendiran, M.; Kumar, J.; Padaria, J.C. Microwave synthesis, characterization and bio-efficacy evaluation of novel chalcone based 6-carbethoxy-2-cyclohexen-1-one and 2H-indazol-3-ol derivatives. Eur. J. Med. Chem. 2013, 59, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T. Sorafenib in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 747s–752s. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Llovet, J.M. Second-line therapies in hepatocellular carcinoma: Emergence of resistance to sorafenib. Clin. Cancer Res. 2012, 18, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, K.; Okamoto, I.; Yonesaka, K.; Hatashita, E.; Yamada, Y.; Fukuoka, M.; Nakagawa, K. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res. 2009, 69, 6515–6521. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W.; Eder, J.P.; Ryan, D.; Lathia, C.; Lenz, H. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin. Cancer Res. 2005, 11, 5472–5480. [Google Scholar] [CrossRef] [PubMed]
- Fieser, L.F.; Peters, M.A. The addition of diazomethane and some of its derivatives to alpha-naphthoquinone. J. Am. Chem. Soc. 1931, 53, 4080–4093. [Google Scholar] [CrossRef]
- Brockmann, H.; Reschks, T. Die struktur der N-methyl-l-in-naphtindazolchinone-(4,9). Tetrahedron Lett. 1965, 50, 4593–4595. [Google Scholar] [CrossRef]
- Eistert, B.; Pfleger, K.; Arackal, T.J.; Holzer, G. Reactions of quinones and α-dicarbonyl compounds with diazoalkanes: XXII Reactions of 2,3-dichloro-p-benzoquinone with diazoalkanes. Chem. Ber. 1975, 108, 693–699. [Google Scholar] [CrossRef]
- Baxter, I.; Davis, B.A. Synthesis of heterocyclic quinones. Q. Rev. Chem. Soc. 1971, 25, 239–263. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Arismendi, M.; Imbarack, E.; Gálvez, C.; Maldonado, J.; San Feliciano, A. The synthesis of some fused pyrazolo-1,4-naphthoquinones. J. Heterocycl. Chem. 2015, 52, 620–622. [Google Scholar] [CrossRef]
- Molinari, A.; Ojeda, C.; Oliva, A.; Miguel del Corral, J.M.; Castro, M.A.; García, P.A.; Cuevas, C.; San Feliciano, A. Synthesis, characterization, and antineoplastic cytotoxicity of hybrid naphthohydroquinone-nucleic base mimic derivatives. Med. Chem. Res. 2009, 18, 59–69. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Ojeda, C.; del Corral, J.M.M.; Castro, M.A.; Molinedo, F.; San Feliciano, A. Synthesis and evaluation as antitumor agents of 1,4-naphthohydroquinone derivatives conjugated with amino acids and purines. Arch. Pharm. Chem. Life Sci. 2009, 346, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.A.; Xu, Y.; Qian, X. Novel naphthalimide-amino acid conjugated with flexible leucine moiety as side chain: design, synthesis and potential antitumor activity. Bioorg. Med. Chem. 2009, 17, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dong, Y.; Gao, J.; Gong, M.; Zhang, X.; Kong, W.; Li, Y.; Zeng, Y.; Si, D.; Wei, Z.; et al. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci. 2015, 106, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kutyrev, A.A. Nucleophilic reactions of quinones. Tetrahedron 1991, 47, 8043–8065. [Google Scholar] [CrossRef]
- Miguel del Corral, J.; Gordaliza, M.; Angeles Castro, M.; Mahiques, M.M.; San Feliciano, A.; García-Grávalos, M.D. Further antineoplastic terpenylquinones and terpenylhydroquinones. Bioorg. Med. Chem. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ros, P. Evaluation of the LCAO Hartree-Fock-Slater method: Applications to transition-metal complexes. Int. J. Quant Chem. 1978, S12, 169–190. [Google Scholar]
- Boerrigter, P.M.; Te Velde, G.; Baerends, J.E. Three-dimensional numerical integration for electronic structure calculations. Int. J. Quant Chem. 1988, 33, 87–113. [Google Scholar]
- Te Velde, G.; Baerends, E.J. Numerical integration for electronic structure calculations. J. Comput. Phys. 1992, 99, 84–98. [Google Scholar] [CrossRef]
- Amsterdam Density Functional (ADF) Program; Vrije Universiteit: Amsterdam, The Netherlands, 2005.
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Versluis, L.; Ziegler, T. The determination of molecular structures by density functional theory. The evaluation of analytical energy gradients by numerical integration. J. Chem. Phys. 1988, 88, 322–329. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2a–c–6a–l are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, A.; Oliva, A.; Arismendi-Macuer, M.; Guzmán, L.; Fuentealba, M.; Knox, M.; Vinet, R.; San Feliciano, A. New 1H-Benzo[f]indazole-4,9-diones Conjugated with C-Protected Amino Acids and Other Derivatives: Synthesis and in Vitro Antiproliferative Evaluation. Molecules 2015, 20, 21924-21938. https://doi.org/10.3390/molecules201219809
Molinari A, Oliva A, Arismendi-Macuer M, Guzmán L, Fuentealba M, Knox M, Vinet R, San Feliciano A. New 1H-Benzo[f]indazole-4,9-diones Conjugated with C-Protected Amino Acids and Other Derivatives: Synthesis and in Vitro Antiproliferative Evaluation. Molecules. 2015; 20(12):21924-21938. https://doi.org/10.3390/molecules201219809
Chicago/Turabian StyleMolinari, Aurora, Alfonso Oliva, Marlene Arismendi-Macuer, Leda Guzmán, Mauricio Fuentealba, Marcela Knox, Raúl Vinet, and Arturo San Feliciano. 2015. "New 1H-Benzo[f]indazole-4,9-diones Conjugated with C-Protected Amino Acids and Other Derivatives: Synthesis and in Vitro Antiproliferative Evaluation" Molecules 20, no. 12: 21924-21938. https://doi.org/10.3390/molecules201219809