Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae)
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemical Study

2.2. Effects of (+)-Phyllanthidine on Host Cell Viability

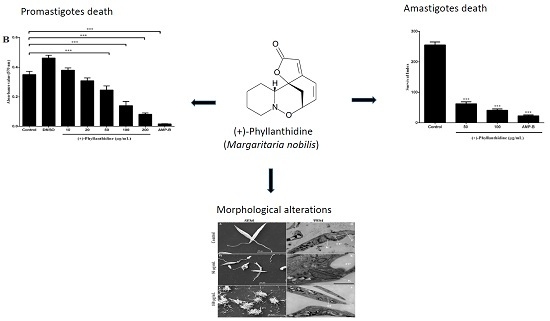
2.3. Effects of (+)-Phyllanthidine on Leishmania (L.) amazonensis Promastigotes and Amastigotes


2.4. Leishmania Ultrastructural Alterations after (+)-Phyllanthidine Treatment

3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Plant Material
3.3. Extraction and Partition
3.4. Isolation and Identification of the Compounds
3.5. Characterization of Compounds 1–6
3.6. (+)-Phyllanthidine Dilution
3.7. Leishmania (L.) amazonensis Parasites
3.8. Peritoneal Macrophage Culture
3.9. Cytotoxicity Assays of Host Cells
3.10. Anti-Promastigote Assay
3.11. Anti-Amastigote Assay
3.12. Ultrastructural Assay
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. World Health Organization (2015). Control of the Leishmaniases. Fact Sheets, 375. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 22 February 2015).
- WHO Expert Committee (2010). Control of the Leishmaniases. WHO Technical Report Series; No. 949. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; Geneva, pp. 22–26. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3307017&tool=pmcentrez&rende rtype=abstract (accessed on 13 March 2010).
- Kedzierski, L. Leishmaniasis. Hum. Vac. 2011, 7, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.L.R.; Jansen, A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy-challenges and opportunities. Clin. Microbiol. Infect. 2011, 17, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, L.; Newton, R. A review of preventative methods against human leishmaniasis infection. PLoS Negl. Trop. Dis. 2013, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Sundar, S.; Dujardin, J.C.; Salotra, P. Elucidation of cellular mechanisms involved in experimental paromomycin resistance in Leishmania donovani. Antimicrob. Agents Chemother. 2014, 58, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mishra, B.B.; Bajpai, S.; Singh, R.K.; Tiwari, V.K. Natural product based leads to fight against leishmaniasis. Bioorganic Med. Chem. 2014, 22, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Kathriarachchi, H.; Hoffmann, P.; Barfuss, M.H.J.; Wurdack, K.J.; Davis, C.C.; Chase, M.W. Molecular phylogenetics of Phyllanthaceae: Evidence from plastid matK and nuclear PHYC sequences. Am. J. Bot. 2005, 92, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Secco, R.S.; Cordeiro, I.; Martins, E.R.; Zappi, D. Phyllanthaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB38479 (accessed on 3 October 2015).
- Arbain, D.; Byrne, L.T.; Cannon, J.R.; Engelhardt, L.M.; White, A.H. The alkaloids of Margaritaria indica (Euphorbiaceae). The crystal structure and absolute configuration of the hydrobromide of (+)-15α-methoxy-14,15-dihydrophyllochrysine. Aust. J. Chem. 1990, 43, 439–445. [Google Scholar] [CrossRef]
- Arbain, D.; Birkbeck, A.A.; Byrne, L.T.; Sargent, M.V.; Skelton, B.W.; White, A.H. The alkaloids of Margaritaria indica. Part 2. The structures of 4-epiphyllanthine, margaritarine and the structural revision of securinol A. J. Chem. Soc. Perkin Trans. 1991, 8, 1863–1869. [Google Scholar] [CrossRef]
- Adepapo, A.A.; Sofidiya, M.O.; Afolayan, A.J. Anti-inflammatory and analgesic activities of the aqueous extracts of Margaritaria discoidea (Euphorbiaceae) stem bark in experimental animal models. Rev. Biol. Trop. 2009, 57, 1193–1200. [Google Scholar]
- Weenen, H.; Nkunya, M.H.H.; Bray, D.H.; Mwasumbi, L.S.; Kinabo, L.S.; Kilimali, V.A.E.B.; Wijnberg, J.B.P.A. Antimalarial compounds containing an α,β-unsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 1990, 56, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.S.T.; Baldé, M.A.; Camara, A.; Traoré, M.S.; Bah, M.L.; Diallo, A.S.; Camara, A.K.; Laurent, S.; Roch, A.; Muller, R.N.; et al. Ethnomedical, phytochemical and biological investigations of Margaritaria discoidea (Ball.) Webster, a plant species widely used in Guinean traditional medicine. J. Plant Sci. 2015, 3, 40–46. [Google Scholar]
- Weinreb, S.M. Total synthesis of the Securinega alkaloids. Nat. Prod. Rep. 2009, 26, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.-Y.; Lan, P.; Sun, P.-H.; Wang, Y.; Ye, W.-C.; Chen, W.-M. Chemical synthesis and biological activities of Securinega alkaloids. J. Chin. Pharm. Sci. 2011, 20, 203–217. [Google Scholar] [CrossRef]
- Kammler, R.; Polborn, K.; Wanner, K.T. Asymmetric synthesis of a tricyclic structure of the Securinega alkaloids virosecurinine and allosecurinine. Tetrahedron 2003, 59, 3359–3368. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Cytotoxic effects of stem bark extracts and pure compounds from Margaritaria discoidea on human ovarian cancer cell lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.S.; Diane, S.; Diallo, M.S.T.; Balde, E.S.; Balde, M.A.; Camara, A.; Diallo, A.; Keita, A.; Cos, P.; Maes, L.; et al. In vitro antiprotozoal and cytotoxic activity of ethnopharmacologically selected Guinean plants. Planta Med. 2014, 80, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Obiri, D.D.; Osafo, N.; Oppong-Sarfo, J.; Prah, J.K. Margaritaria discoides (Euphorbiaceae) stem bark extract attenuates allergy and Freund’s adjuvant-induced arthritis in rodents. Pharmacogn. Res. 2014, 6, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Cho-Ngwa, F.; Abongwa, M.; Ngemenya, M.N.; Nyongbela, K.D. Selective activity of extracts of Margaritaria discoidea and Homalium africanum on Onchocerca ochengi. BMC Complement. Altern. Med. 2010, 10, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.I.; Romero, L.I.; Ortega-Barría, E.; Brun, R.; Correa A, M.D.; Gupta, M.P. Evaluation of larvicidal in vitro antiparasitic activities of plants in a biodiversity plot in the Altos de Campana National Park, Panama. Pharm. Biol. 2006, 44, 487–498. [Google Scholar] [CrossRef]
- Parello, J.; Munavalli, S. Phyllanthine et phyllanthidine, alcaloides du Phyllanthus discoides Müll. Arg. (Euphorbiacées). C. R. Acad. Sci. 1965, 260, 337–340. [Google Scholar]
- Horii, Z.; Imanishi, T.; Yamauchi, M.; Hanaoka, M. Structure of phyllanthidine. Tetrahedron Lett. 1972, 19, 1877–1880. [Google Scholar] [CrossRef]
- Lajis, N.H.; Guan, O.B.; Sargent, M.V.; Skelton, B.W.; White, A.H. Viroallosecurinine and ent-phyllanthidine from the leaves of Breynia coronata (Euphorbiaceae). Aust. J. Chem. 1992, 45, 1893–1897. [Google Scholar]
- Carson, C.A.; Kerr, M.A. Total synthesis of (+)-phyllantidine. Angew. Chem. Int. Ed. 2006, 45, 6560–6563. [Google Scholar] [CrossRef] [PubMed]
- Marín, C.; Boutaleb-Charki, S.; Díaz, J.G.; Huertas, O.; Rosales, M.J.; Pérez-Cordon, G.; Gutierrez-Sánchez, R.; Sánchez-Moreno, M. Antileishmaniasis activity of flavonoids from Consolida oliveriana. J. Nat. Prod. 2009, 72, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Radtke, O.A.; Kiderlen, A.F. Stimulus (polyphenol, IFN-γ, LPS)-dependent nitric oxid production and antileishmanial effects in RAW 264.7 macrophages. Phytochemistry 2008, 69, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Burmeister, A.; Trun, W.; Radtke, O.A.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L.Y. Tannins and related compounds induce nitric oxide synthase and cytokines gene expression in Leishmania major-infected macrophage-like RAW 264.7 cells. Bioorg. Med. Chem. 2005, 13, 6470–6476. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Carmona, D.B.; Escalante-Erosa, F.; García-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D.; Chan-Bacab, M.J.; Giménez-Turba, A.; Peña-Rodríguez, L.M. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Alavi-naini, R.; Fazaeli, A.; O’Dempsey, T. Tropical treatment modalities for old world cutaneous leishmaniasis: A review. Prague Med. Rep. 2012, 113, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Delorenzi, J.C.; Attias, M.; Gattas, C.R.; Andrade, M.; Rezende, C.; Pinto, A.C.; Henriques, A.T.; Bou-Habib, D.C.; Saraiva, E.M.B. Antileishmanial activity of an indole alkaloid from Peschiera australis. Antimicrob. Agents Chemother. 2001, 45, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.; Rodrigues, A.P.D.; Marinho, P.S.B.; Müller, A.H.; Guilhon, G.M.S.P.; Santos, L.S.; Do Nascimento, J.L.N.; Silva, E.O. Activity of julocrotine, a glutarimide alkaloid from Croton pullei var. glabrior, on Leishmania (L.) amazonensis. Parasitol. Res. 2010, 107, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Kima, P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007, 37, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive oxygen species production by quercetin causes the death of Leishmania amazonensis intracellular amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.D.; Farias, L.H.S.; Carvalho, A.S.C.; Santos, A.S.; Do Nascimento, J.L.M.; Silva, E.O. A novel function for kojic acid, a secondary metabolite from Aspergillus fungi, as antileishmanial agent. PLoS ONE 2014, 9, e91259. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.P.; Silva, B.J.M.; Rodrigues, A.P.D.; Farias, L.H.S.; Silva, M.N.; Alves, D.T.V.; Bastos, G.N.T.; Do Nascimento, J.L.M.; Silva, E.O. In vitro biological action of aqueous extract from roots of Physalis angulata against Leishmania (Leishmania) amazonensis. BMC Complement. Altern. Med. 2015, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.C.; Rocha, V.P.; Nonato, F.R.; Tomassini, T.C.; Ribeiro, I.M.; Santos, R.; Soares, M.B. Genotoxicity and antileishmanial activity evaluation of Physalis angulata concentrated ethanolic extract. Environ. Toxicol. Pharmacol. 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Marín, C.; Rodrıguez-Gonzalez, I.; Hitos, A.B.; Rosales, M.J.; Reina, M.; Díaz, J.G.; González-Coloma, A.; Sánchez-Moreno, M. In vitro activity of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents 2005, 25, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Vannier-Santos, M.A.; de Castro, S.L. Electron microscopy in antiparasitic chemotherapy: A (close) view to a kill. Curr. Drug Targets 2009, 10, 246–260. [Google Scholar] [CrossRef]
- Adade, C.M.; Souto-Padron, T. Contributions of ultrastructural studies to the cell biology of trypanosmatids: Targets for anti-parasitic drugs. Open Parasitol. 2010, 4, 178–187. [Google Scholar] [CrossRef]
- Ueda-Nakamura, T.; Mendonça-Filho, R.R.; Morgado-Diáz, J.A.; Maza, P.K.; Dias Filho, B.P.; Cortez, D.A.G.; Alviano, D.S.; Rosa, M.S.S.; Lopes, A.H.C.S; Alviano, C.S.; et al. Antileishmanial activity of eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine 2011, 18, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.R.G.; Esteves-Souza, A.; Vieira, I.J.C.; Mathias, L.; Braz-Filho, R.; Echevarria, A. Flavonoids from Annona dioica leaves and their effects in Ehrlich carcinoma cells, DNA-topoisomerase I and II. J. Braz. Chem. Soc. 2007, 18, 1554–1559. [Google Scholar] [CrossRef]
- Hisham, D.M.N.; Lip, J.M.; Noh, J.M.; Norman, A.; Nabilah, M.F.N. Identification and isolation as a polar chemical marker for Labisia pumila Benth. J. Trop. Agric. Food Sci. 2011, 39, 279–284. [Google Scholar]
- Zhang, Y.; DeWitt, D.L.; Murugesan, S.; Nair, M.G. Novel lipid-peroxidation- and cyclooxygenase-inhibitory tannins from Picrorhiza kurroa seeds. Chem. Biodivers. 2004, 1, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Conegero, L.S.; Ide, R.M.; Nazari, A.S.; Sarragiootto, M.H.; Dias Filho, B.P.; Nakamura, C.V.; Carvalho, J.E.; Foglio, M.A. Constituintes químicos de Alchornea glandulosa (Euphorbiaceae). Quím. Nova 2003, 26, 825–827. [Google Scholar] [CrossRef]
- Bisoli, E.; Garcez, W.S.; Hamerski, L.; Tieppo, C.E.; Garcez, F.R. Bioactive pentacyclic triterpenes from the stems of Combretum laxum. Molecules 2008, 13, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Atta-ur-Rahman. Handbook of Natural Products Data. Pentacyclic Triterpenoids; Elsevier: Amsterdam, The Netherlands, 1994; Volume 2, pp. 1102–1103. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of compounds 1–6 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, L.S.; Donza, M.R.H.; Rodrigues, A.P.D.; Silva, B.J.M.; Brasil, D.S.B.; Zoghbi, M.D.G. B.; Andrade, E.H.A.; Guilhon, G.M.S.P.; Silva, E.O. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae). Molecules 2015, 20, 22157-22169. https://doi.org/10.3390/molecules201219829
Moraes LS, Donza MRH, Rodrigues APD, Silva BJM, Brasil DSB, Zoghbi MDGB, Andrade EHA, Guilhon GMSP, Silva EO. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae). Molecules. 2015; 20(12):22157-22169. https://doi.org/10.3390/molecules201219829
Chicago/Turabian StyleMoraes, Lienne S., Marcio R. H. Donza, Ana Paula D. Rodrigues, Bruno J. M. Silva, Davi S. B. Brasil, Maria Das Graças B. Zoghbi, Eloísa H. A. Andrade, Giselle M. S. P. Guilhon, and Edilene O. Silva. 2015. "Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae)" Molecules 20, no. 12: 22157-22169. https://doi.org/10.3390/molecules201219829