Pharmacokinetics and Tissue Distribution Study of Caudatin in Normal and Diethylnitrosamine-Induced Hepatocellular Carcinoma Model Rats
Abstract
:1. Introduction
2. Results and Discussion
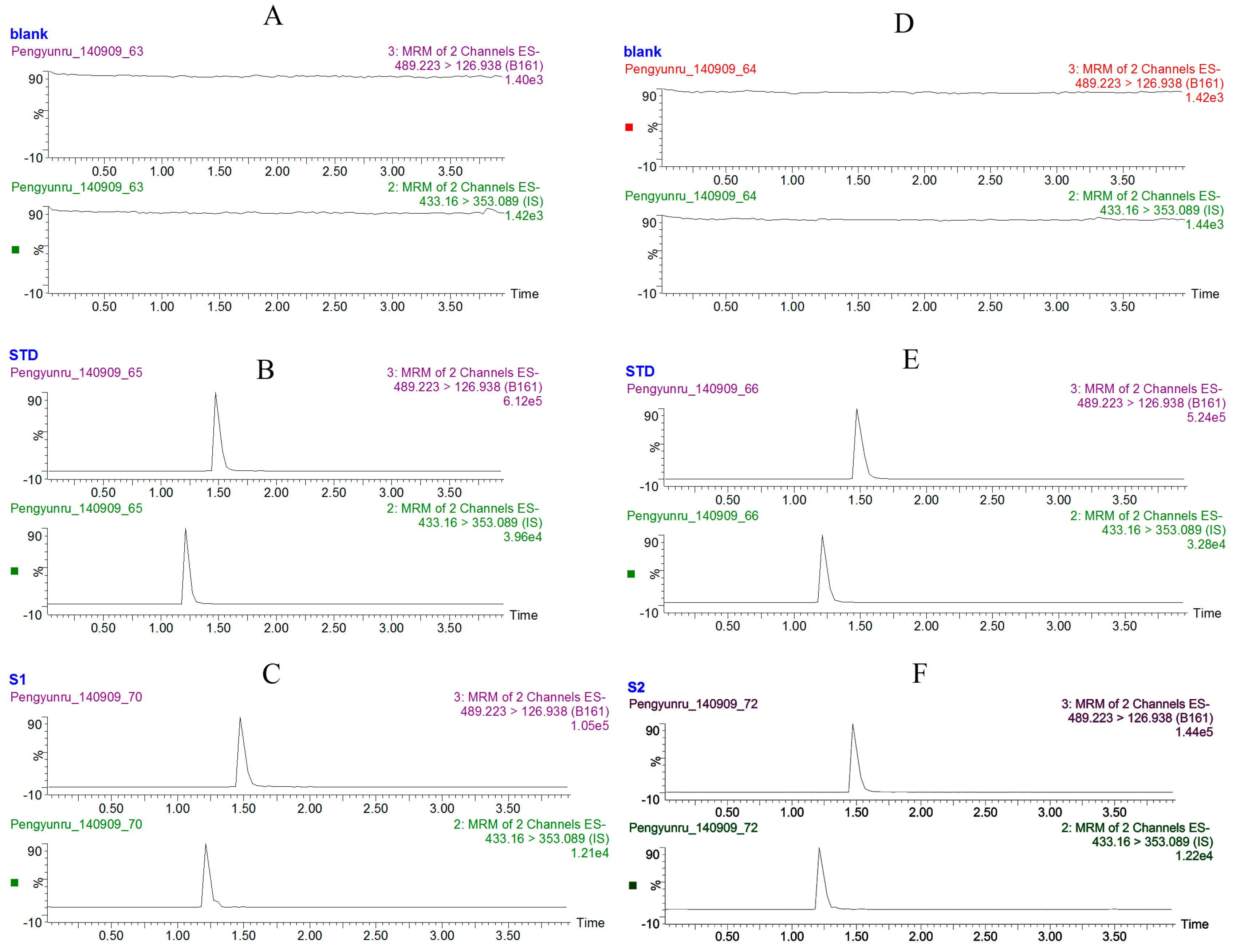
2.1. Method Development
2.2. Method Validation
| Concentration (ng/mL) | Intra-day R.S.D. (%) | Inter-day R.S.D. (%) | Accuracy R.E. (%) | Recovery (%) | |
|---|---|---|---|---|---|
| plasma | 10 | 9.5 | 9.2 | 6.6 | 95.6 |
| 100 | 6.8 | 8.4 | −5.5 | 96.8 | |
| 800 | 7.6 | 6.5 | 8.7 | 92.4 | |
| tissue homogenate | 10 | 6.9 | 7.7 | −8.9 | 99.3 |
| 100 | 8.7 | 9.4 | 7.1 | 91.1 | |
| 800 | 8.6 | 9.5 | 5.5 | 93.1 |
2.3. Method Application
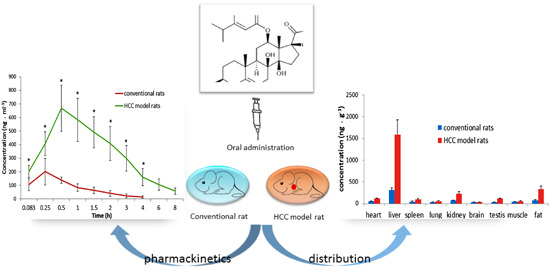
2.3.1. Pharmacokinetic Study
| Stability | Added C (ng/mL) | Found C (ng/mL) | Accuracy R.E. (%) | |
|---|---|---|---|---|
| plasma | 48 h at room temperature | 10 | 9.1 | −9.0 |
| 100 | 95.7 | −4.3 | ||
| 800 | 789.4 | −1.3 | ||
| 1 month at −20 °C | 10 | 9.6 | −4.0 | |
| 100 | 96.2 | −3.8 | ||
| 800 | 814.2 | 1.8 | ||
| three freeze-thaw cycles | 10 | 10.4 | 4.0 | |
| 100 | 94.2 | −5.8 | ||
| 800 | 759.1 | −5.1 | ||
| tissue homogenate | 48 h at room temperature | 10 | 10.3 | 3.0 |
| 100 | 92.3 | −7.7 | ||
| 800 | 745.5 | −6.8 | ||
| 1 month at −20 °C | 10 | 9.2 | −8.0 | |
| 100 | 93.2 | −6.8 | ||
| 800 | 736.6 | −7.9 | ||
| three freeze–thaw cycles | 10 | 9.4 | −6.0 | |
| 100 | 105.4 | 5.4 | ||
| 800 | 857.3 | 7.2 |

| Parameter | Unit | Conventional Rats | HCC Model Rats |
|---|---|---|---|
| AUC(0-t) | µg·h/L | 236.8 ± 62.8 | 2006.0 ± 334.1 * |
| AUC(0-∞) | µg·h/L | 263.4 ± 76.2 | 2251.9 ± 386.0 * |
| MRT(0-t) | h | 1.21 ± 0.13 | 2.47 ± 0.32 * |
| MRT(0-∞) | h | 1.68 ± 0.39 | 3.62 ± 1.11 * |
| T1/2z | h | 1.25 ± 0.49 | 2.89 ± 1.78 * |
| Tmax | h | 0.29 ± 0.10 | 1.00 ± 0.45 * |
| Cmax | µg/L | 314.7 ± 82.0 | 713.4 ± 129.3 * |
| CLz/F | L/h/kg | 80.8 ± 20.8 | 9.13 ± 1.75 * |
| Vz/F | L/kg | 147.7 ± 78.0 | 37.2 ± 21.2 * |
2.3.2. Tissue Distribution Study

| Plasma or Tissues | Fold Increase | ||
|---|---|---|---|
| 0.5 h | 1.0 h | 3.0 h | |
| plasma | 4.83 | 6.95 | 13.36 |
| heart | 2.91 | 3.37 | 2.23 |
| liver | 4.26 | 4.30 | 5.10 |
| spleen | 4.63 | 4.26 | 2.31 |
| lung | 1.83 | 1.82 | 1.65 |
| kidney | 4.18 | 4.19 | 3.05 |
| brain | 1.48 | 1.56 | 1.05 |
| testis | 13.89 | 7.90 | 3.38 |
| muscle | 0.94 | 1.09 | 1.30 |
| fat | 5.95 | 3.96 | 4.46 |
| stomach | 0.95 | 0.95 | 0.88 |
| small intestine | 0.84 | 0.88 | 0.93 |
| rectum | 0.97 | 1.06 | 0.93 |
3. Experimental
3.1. Materials, Reagents and Animals


3.2. Instrumentation and Conditions
3.3. Standard Solution and Quality Control Samples
3.4. Sample Preparation
3.5. Method Validation
3.6. Application to Pharmacokinetics and Tissue Distributions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Center, M.M.; Jemal, A. International trends in liver cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.L.; Li, X.; Zhang, S.X.; Zhang, H.Q. Protective effect of C21 steroidal ester saponin of Cynanchum auriculatum royle ex wight on acute CCL4- induced liver injury in mice. Anhui Med. Pharm. J. 2007, 11, 198–200. [Google Scholar]
- Lv, W.H.; Zhang, A.X.; Xu, S.; Zhang, H.Q. Effects of general glycosides in Cynanchum auriculatum of Jiangsu province on liver fibrosis of rats. China J. Chin. Mater. Med. 2009, 34, 2508–2511. [Google Scholar]
- Wang, D.Y.; Zhang, H.Q.; Li, X. Apoptosis induced by the C21 sterols in Baishouwu and its mechanism of action in hepatoma. Acta Pharm. Sin. 2007, 42, 366–370. [Google Scholar]
- Bi, F.; Tao, W.Y.; Lu, Z.M. Anti-tumor efficacy of the extract from Radix Cynanchi Auriculati on mouse liver cancer Hepatoma 22. Chin. Tradit. Pat. Med. 2007, 29, 1586–1590. [Google Scholar]
- Bi, F.; Guo, W.J.; Tao, W.Y. Screening of bioactive substances from the root of Cynanchum Auriculatum Royle ex Wight by Anti-Liver Cancer Bel 7402 in vitro. J. Food Sci. Biotechnol. 2008, 27, 109–112. [Google Scholar]
- Peng, Y.R.; Li, Y.B.; Liu, X.D.; Zhang, J.F.; Duan, J.A. Antitumor activity of C-21 steroidal glycosides from Cynanchum auriculatum Royle ex Wight. Phytomedicine 2008, 15, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.R.; Li, Y.B.; Liu, X.D.; Zhang, J.F.; Duan, J.A. Apoptosis induced by caudatin in human hepatoma cell line SMMC7721. Chin. J. Nat. Med. 2008, 6, 210–213. [Google Scholar] [CrossRef]
- Fei, H.R.; Chen, H.L.; Xiao, T.; Chen, G.; Wang, F.Z. Caudatin induces cell cycle arrest and caspase- dependent apoptosis in HepG2 cell. Mol. Biol. Rep. 2012, 39, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Geng, C.A.; Ma, Y.B.; Luo, J.; Huang, X.Y.; Chen, H.; Zhou, N.J.; Zang, X.M.; Chen, J.J. Design, synthesis and molecular hybrids of caudatin and cinnamic acids as novel anti- hepatitis B virus agents. Eur. J. Med. Chem. 2012, 54, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Geng, C.A.; Ma, Y.B.; Huang, X.Y.; Luo, J.; Chen, H.; Guo, R.H.; Zhang, X.M.; Chen, J.J. Synthesis, structure- activity relationships and biological evaluation of caudatin derivatives as novel anti- hepatitis B virus agents. Bioorg. Med. Chem. 2012, 20, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.R.; Wang, D.W.; Ding, Y.F.; Luo, Y.H.; Li, Y.B.; Liu, X.D. Oral pharmacokinetics of Caudatin in rats. Chin. J. Nat. Med. 2010, 8, 471–473. [Google Scholar]
- Wang, D.W.; Peng, Y.R.; Li, Y.B.; Qian, S.H.; Wu, J.; Zhang, J. Study on determination of caudatin in rat plasma by LC-MS-MS. Chin. Pharm. J. 2008, 43, 704–706. [Google Scholar]
- Yan, T.; Gao, S.; Peng, X.; Shi, J.; Xie, C.; Li, Q.; Lu, L.; Wang, Y.; Zhou, F.; Liu, Z.; et al. Significantly decreased and more variable expression of major CYPs and UGTs in liver microsomes prepared from HBV-positive human hepatocellular carcinoma and matched pericarcinomatous tissues determined using an isotope label-free UPLC-MS/MS method. Pharm. Res. 2015, 32, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Ebara, M.; Okuyama, M.; Sugiura, N.; Yoshikawa, M.; Saisho, H.; Shimizu, R.; Motoji, N.; Shigematsu, A.; Watayo, T. Increased metabolizing activities of the tricarboxylic acid cycle and decreased drug metabolism in hepatocellular carcinoma. Carcinogenesis 2002, 23, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Fritz, P.; Behrle, E.; Beaune, P.; Eichelbaum, M.; Kroemer, H.K. Differential expression of drug metabolizing enzymes in primary and secondary liver neoplasm: Immunohistochemical characterization of cytochrome P4503A and glutathione-S-transferase. Histochemistry 1993, 99, 443–451. [Google Scholar] [PubMed]
- Ye, L.; Yang, X.S.; Guo, E.S.; Chen, W.Y.; Lu, L.L.; Wang, Y.; Peng, X.J.; Yan, T.M.; Zhou, F.Y.; Liu, Z.Q. Sorafenib metabolism is significantly altered in the liver tumor tissue of hepatocellular carcinoma patient. PLoS One 2014, 9, e96664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Zha, B.S.; Zhang, H.L.; Zhu, X.J.; Li, Y.H.; Zhu, L.; Guan, X.H.; Feng, Z.Q.; Zhang, J.P. Characteristic gene expression profiles in the progression from liver cirrhosis to carcinoma induced by diethylnitrosamine in a rat mode. J. Exp. Clin. Cancer Res. 2009, 28, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Roy, S.; Chatterjee, M. Characterization of selective induction and alteration of xenobiotic biotransforming enzymes by vanadium during diethylnitrosamine-induced chemical rat liver carcinogenesis. Oncol. Res. 1999, 11, 41–53. [Google Scholar] [PubMed]
- Rui, W.J.; He, S.F.; Huang, X.J.; Fang, Z.; Hang, X.; Li, Q.J.; Hu, M.; Yang, Y. Expression of pSmad3L and PAI-1 during the process of DEN-induced hepatic fibrosis-carcinoma in rats. Chin. Pharmacol. Bull. 2012, 28, 1393–1397. [Google Scholar]
- Sample Availability: Samples of the compound caudatin are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Ding, Y. Pharmacokinetics and Tissue Distribution Study of Caudatin in Normal and Diethylnitrosamine-Induced Hepatocellular Carcinoma Model Rats. Molecules 2015, 20, 4225-4237. https://doi.org/10.3390/molecules20034225
Peng Y, Ding Y. Pharmacokinetics and Tissue Distribution Study of Caudatin in Normal and Diethylnitrosamine-Induced Hepatocellular Carcinoma Model Rats. Molecules. 2015; 20(3):4225-4237. https://doi.org/10.3390/molecules20034225
Chicago/Turabian StylePeng, Yunru, and Yongfang Ding. 2015. "Pharmacokinetics and Tissue Distribution Study of Caudatin in Normal and Diethylnitrosamine-Induced Hepatocellular Carcinoma Model Rats" Molecules 20, no. 3: 4225-4237. https://doi.org/10.3390/molecules20034225