Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Flower Scent Volatiles Extraction
2.2. Optimization of HS-SPME Parameters
| Code | Sample Weight (A) | Extraction Time (B) | Handle Scale (C) | Desorption Temperature (D) | Peak Area |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 2.09 × 109 |
| 2 | 1 | 2 | 2 | 2 | 1.49 × 109 |
| 3 | 1 | 3 | 3 | 3 | 1.36 × 109 |
| 4 | 1 | 4 | 4 | 4 | 1.01 × 109 |
| 5 | 2 | 1 | 2 | 4 | 2.90 × 109 |
| 6 | 2 | 2 | 1 | 3 | 2.55 × 109 |
| 7 | 2 | 3 | 4 | 2 | 3.22 × 109 |
| 8 | 2 | 4 | 3 | 1 | 1.54 × 109 |
| 9 | 3 | 1 | 3 | 2 | 2.01 × 109 |
| 10 | 3 | 2 | 4 | 1 | 1.27 × 109 |
| 11 | 3 | 3 | 1 | 4 | 2.09 × 109 |
| 12 | 3 | 4 | 2 | 3 | 3.31 × 109 |
| 13 | 4 | 1 | 4 | 3 | 1.34 × 109 |
| 14 | 4 | 2 | 3 | 4 | 3.95 × 109 |
| 15 | 4 | 3 | 2 | 1 | 8.50 × 108 |
| 16 | 4 | 4 | 1 | 2 | 9.70 × 108 |
| Mean value 1 | 1.49 × 109 | 2.09 × 109 | 1.93 × 109 | 1.44 × 109 | |
| Mean value 2 | 2.55 × 109 | 2.32 × 109 | 2.14 × 109 | 1.92 × 109 | |
| Mean value 3 | 2.17 × 109 | 1.88 × 109 | 2.22 × 109 | 2.14 × 109 | |
| Mean value 4 | 1.78 × 109 | 1.71 × 109 | 1.71 × 109 | 2.49 × 109 | |
| Range | 1.07 × 109 | 6.08 × 108 | 5.05 × 108 | 1.05 × 109 | |
| Optimization level | A2 | B2 | C3 | D4 |
2.3. Quantitative Evaluation of Floral Scent Volatile Compounds

2.4. Volatile Compounds of Chrysanthemum and Its Wild Relatives
| Quantity | 10–20 | 20–29 | 30–39 | ≥40 |
|---|---|---|---|---|
| Plant accessions | 9 | 19 | 8 | 2 |
| Percent | 24% | 50% | 21% | 5% |
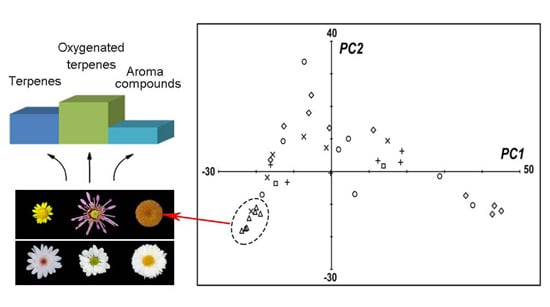
2.5. Patterns of Floral Scent Volatiles in Accessions of Chrysanthemum and Its Relatives



3. Experimental Section
3.1. Plant Materials
| Code | Accessions | Collection Locality |
|---|---|---|
| 1 | Chrysanthemum morifolium “Chi huang” | Nanjing, Jiangsu province, China |
| 2 | Chrysanthemum morifolium “Chu ju” | Nanjing, Jiangsu province, China |
| 3 | Chrysanthemum morifolium “Hang bai ju” | Nanjing, Jiangsu province, China |
| 4 | Chrysanthemum morifolium “Nan nong cai feng che” | Nanjing, Jiangsu province, China |
| 5 | Chrysanthemum morifolium “Nan nong fei zi” | Nanjing, Jiangsu province, China |
| 6 | Chrysanthemum morifolium “Nan nong fen feng che” | Nanjing, Jiangsu province, China |
| 7 | Chrysanthemum morifolium “Nan nong hua feng che” | Nanjing, Jiangsu province, China |
| 8 | Chrysanthemum morifolium “Nan nong huang kou” | Nanjing, Jiangsu province, China |
| 9 | Chrysanthemum morifolium “Nan nong huo ju” | Nanjing, Jiangsu province, China |
| 10 | Chrysanthemum morifolium “Nan nong jin kou” | Nanjing, Jiangsu province, China |
| 11 | Chrysanthemum morifolium “Nan nong jin rong” | Nanjing, Jiangsu province, China |
| 12 | Chrysanthemum morifolium “Nan nong jin hong” | Nanjing, Jiangsu province, China |
| 13 | Chrysanthemum morifolium “Nan nong ju yu” | Nanjing, Jiangsu province, China |
| 14 | Chrysanthemum morifolium “Nan nong li cui” | Nanjing, Jiangsu province, China |
| 15 | Chrysanthemum morifolium “Nan nong mei fen” | Nanjing, Jiangsu province, China |
| 16 | Chrysanthemum morifolium “Nan nong qiao feng che” | Nanjing, Jiangsu province, China |
| 17 | Chrysanthemum morifolium “Nan nong wu feng che” | Nanjing, Jiangsu province, China |
| 18 | Chrysanthemum morifolium “Nan nong xia yu” | Nanjing, Jiangsu province, China |
| 19 | Chrysanthemum morifolium “Nan nong xiang bin” | Nanjing, Jiangsu province, China |
| 20 | Chrysanthemum morifolium “Nan nong xiao li” | Nanjing, Jiangsu province, China |
| 21 | Chrysanthemum morifolium “Nan nong xuan cai” | Nanjing, Jiangsu province, China |
| 22 | Chrysanthemum morifolium “Nan nong xuan kui” | Nanjing, Jiangsu province, China |
| 23 | Chrysanthemum morifolium “Nan nong xue rong” | Nanjing, Jiangsu province, China |
| 24 | Chrysanthemum morifolium “Nan nong yu quan” | Nanjing, Jiangsu province, China |
| 25 | Chrysanthemum morifolium “Nan nong yu rong” | Nanjing, Jiangsu province, China |
| 26 | Chrysanthemum morifolium “Lv fengche” | Nanjing, Jiangsu province, China |
| 27 | Chrysanthemum morifolium “Yi dian hei” | Nanjing, Jiangsu province, China |
| 28 | Chrysanthemum morifolium “Zhe jiang 12” | Nanjing, Jiangsu province, China |
| 29 | Chrysanthemum morifolium “Nan nong zi xing” | Nanjing, Jiangsu province, China |
| 30 | Ajania marginatum | Tsukuba, Japan |
| 31 | Ajania myriantha | Jinchuan, Sichuan province, China |
| 32 | Ajania shiwogiku | Tsukuba, Japan |
| 33 | Chrysanthemum boreale | Tsukuba, Japan |
| 34 | Chrysanthemum indicum | Nanjing, Jiangsu province, China |
| 35 | Chrysanthemum indicum | Hangzhou, Zhejiang province China |
| 36 | Chrysanthemum indicum | Huangshan, Anhui province, China |
| 37 | Chrysanthemum japonense | Tokyo, Japan |
| 38 | Chrysanthemum nankingense | Nanjing, Jiangsu province, China |
| 39 | Chrysanthemum yoshinaganthum | Tsukuba, Japan |
3.2. Sample Preparation
3.3. Optimization of HS-SPME Parameters
| Level | Factor | |||
|---|---|---|---|---|
| Sample Weight (A/g) | Extraction Time (B/min) | Handle Graduation (C/cm) | Desorption Temperature (D/°C) | |
| 1 | 2.5 | 40 | 1 | 220 |
| 2 | 2.0 | 30 | 2 | 230 |
| 3 | 1.5 | 20 | 3 | 240 |
| 4 | 1.0 | 10 | 4 | 250 |
3.4. GC-MS Conditions
3.5. Peak Identification
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Azam, M.; Song, M.; Fan, F.; Zhang, B.; Xu, Y.; Xu, C.; Chen, K. Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages. Int. J. Mol. Sci. 2013, 14, 22346–22367. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.M. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution 2000, 54, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.L.; Snow, A.A.; Handel, S.N. Floral evolution: Attractiveness to pollinators increases male fitness. Science 1986, 232, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Effmert, U. Biosynthesis and Regulation of Flower Scent; Springer: Berlin/Heidelberg, Germany, 2010; pp. 189–205. [Google Scholar]
- Wäckers, F. Assessing the suitability of flowering herbs as parasitoid food sources: Flower attractiveness and nectar accessibility. Biol. Control 2004, 29, 307–314. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Song, A.; Zhu, X.; Chen, F.; Gao, H.; Jiang, J.; Chen, S. A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int. J. Mol. Sci. 2014, 15, 5063–5078. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Shinoyama, H.; Aida, R.; Matsushita, Y.; Raj, S.K.; Chen, F. Chrysanthemum Biotechnology: Quo vadis? Crit. Rev. Plant Sci. 2013, 32, 21–52. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower Chrysanthemum morifolium Ramat. Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, L.; Challis, R.; Leyser, O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J. Exp. Bot. 2010, 61, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, Y.; Yu, H.; Ying, Y.; Zou, G. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.D. Antioxidant activity of water extract of four Harng Jyur Chrysanthemum morifolium Ramat varieties in soybean oil emulsion. Food Chem. 1999, 66, 471–476. [Google Scholar] [CrossRef]
- Woo, K.; Yu, J.; Hwang, I.; Lee, Y.; Lee, C.; Yoon, H.; Lee, J.; Jeong, H. Antioxidative activity of volatile compounds in flower of Chrysanthemum indicum, C. morifolium, and C. zawadskii. J. Korean Soc. Food Sci. Nutr. 2008, 37, 805–809. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Zhang, L.; Xiao, S. Studies on chemical constituents from flowers of Chrysanthemum indicum. China J. Chin. Mater. Med. 2008, 31, 682–684. [Google Scholar]
- Boutaghane, N.; Kabouche, A.; El Azzouny, A.; Kabouche, Z. Composition of the essential oil of Chrysanthemum macrocarpum from Algeria. Chem. Nat. Compd. 2008, 44, 817–818. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, P.; Wang, W.; Ma, J.; Li, P. Analysis of volatile components from the flowers of Chrysanthemum morifolium by GC-MS with solid-phase microextraction. China J. Chin. Mater. Med. 2005, 30, 986–989. [Google Scholar]
- Chang, K.M.; Kim, G.H. Volatile aroma composition of Chrysanthemum indicum L. flower oil. J. Food Sci. Nutr. 2008, 13, 122–127. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, M.J.; Shu, P.; Hong, J.L.; Lü, L.; He, D.X. Chemical variations of the essential oils in flower heads of Chrysanthemum indicum L. from China. Chem. Biodivers. 2010, 7, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.M.; Kim, G.H. Volatile aroma constituents of gukhwa (Chrysanthemum morifolium R.). Food Sci. Biotechnol. 2013, 22, 659–663. [Google Scholar] [CrossRef]
- Prosen, H.; Zupančič Kralj, L. Solid-phase microextraction. Trac. Trend Anal. Chem. 1999, 18, 272–282. [Google Scholar] [CrossRef]
- Deng, C.; Xu, X.; Yao, N.; Li, N.; Zhang, X. Rapid determination of essential oil compounds in Artemisia Selengensis Turcz by gas chromatography-mass spectrometry with microwave distillation and simultaneous solid-phase microextraction. Anal. Chim. Acta 2006, 556, 289–294. [Google Scholar] [CrossRef]
- Chen, H.C.; Chi, H.S.; Lin, L.Y. Headspace Solid-Phase Microextraction Analysis of Volatile Components in Narcissus tazetta var. chinensis Roem. Molecules 2013, 18, 13723–13734. [Google Scholar] [CrossRef]
- Li, Z.G.; Lee, M.R.; Shen, D.L. Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different florescence by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Am. J. Transpl. 2006, 576, 43–49. [Google Scholar]
- Li, S.S.; Chen, L.G.; Xu, Y.J.; Wang, L.J.; Wang, L.S. Identification of floral fragrances in tree peony cultivars by gas chromatography-mass spectrometry. Sci. Hortic. 2012, 142, 158–165. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, C.; Song, G.; Hu, Y. Comparison of essential oil composition of Artemisia argyi leaves at different collection times by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Chromatographia 2004, 59, 729–732. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Prades, A.; Assa, R.R.A.; Dornier, M.; Pain, J.P.; Boulanger, R. Characterisation of the volatile profile of coconut water from five varieties using an optimised HS—SPME—GC analysis. J. Sci. Food Agric. 2012, 92, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, J.; Deng, C.; Shen, X. Gas chromatography—Mass spectrometry following pressurized hot water extraction and solid—Phase microextraction for quantification of eucalyptol, camphor, and borneol in Chrysanthemum flowers. J. Sep. Sci. 2007, 30, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Palomo, E.; Diaz Maroto, M.C.; Pérez Coello, M.S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC–MS. Talanta 2005, 66, 1152–1157. [Google Scholar]
- Basta, A.; Pavlović, M.; Couladis, M.; Tzakou, O. Essential oil composition of the flowerheads of Chrysanthemum coronarium L. from Greece. Flavour Frag. J. 2007, 22, 197–200. [Google Scholar] [CrossRef]
- Song, A.A.L.; Abdullah, J.O.; Abdullah, M.P.; Shafee, N.; Rahim, R.A. Functional expression of an orchid fragrance gene in Lactococcus lactis. Int. J. Mol. Sci. 2012, 13, 1582–1597. [Google Scholar] [CrossRef]
- Yasukawa, K.; Akihisa, T.; Kasahara, Y.; Ukiya, M.; Kumaki, K.; Tamura, T.; Yamanouchi, S.; Takido, M. Inhibitory Effect of Stevioside on Tumor Promotion by 12-O-Tetradecanoylphorbol-13-acetate in Two-Stage Carcinogenesis in Mouse Skin. Phytomedicine 1998, 5, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Takasaki, M.; Ichiishi, E.; Murakami, T.; Tokuda, H.; Nishino, H.; Duc, N.M.; Kasai, R.; Yamasaki, K. Cancer chemopreventive activity of majonoside-R2 from Vietnamese ginseng, Panax vietnamensis. Cancer Lett. 1999, 147, 11–16. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, T.; Fan, Q.; Qi, X.; Zhang, F.; Fang, W.; Jiang, J.; Chen, F.; Chen, S. Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry. Molecules 2015, 20, 5346-5359. https://doi.org/10.3390/molecules20045346
Sun H, Zhang T, Fan Q, Qi X, Zhang F, Fang W, Jiang J, Chen F, Chen S. Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry. Molecules. 2015; 20(4):5346-5359. https://doi.org/10.3390/molecules20045346
Chicago/Turabian StyleSun, Hainan, Ting Zhang, Qingqing Fan, Xiangyu Qi, Fei Zhang, Weimin Fang, Jiafu Jiang, Fadi Chen, and Sumei Chen. 2015. "Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry" Molecules 20, no. 4: 5346-5359. https://doi.org/10.3390/molecules20045346
APA StyleSun, H., Zhang, T., Fan, Q., Qi, X., Zhang, F., Fang, W., Jiang, J., Chen, F., & Chen, S. (2015). Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry. Molecules, 20(4), 5346-5359. https://doi.org/10.3390/molecules20045346