Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

2.2. Anti-Inflammatory Activity
| Groups | Increase in Paw Volume (Edema Volume) (mL) | |||
|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | |
| Control | 0.42 ± 0.01 | 0.71 ± 0.01 | 0.8 ± 0.01 | 0.84 ± 0.01 |
| Indomethacin (10 mg/kg) | 0.45 ± 0.01 | 0.65 ± 0.02 | 0.60 ± 0.02 *** | 0.56 ± 0.02 *** |
| 1 | 0.44 ± 0.03 | 0.66 ± 0.03 | 0.72 ± 0.03 | 0.64 ± 0.03 *** |
| 2 | 0.43 ± 0.02 | 0.58 ± 0.02 ** | 0.68 ± 0.02 ** | 0.61 ± 0.02 *** |
| 3 | 0.46 ± 0.02 | 0.56 ± 0.02 *** | 0.66 ± 0.02 *** | 0.57 ± 0.02 *** |
| 4 | 0.40 ± 0.02 | 0.50 ± 0.02 *** | 0.57 ± 0.02 *** | 0.47 ± 0.02 *** |
| 5 | 0.46 ± 0.03 | 0.63 ± 0.03 | 0.70 ± 0.03 ** | 0.60 ± 0.03 *** |
| 6 | 0.41 ± 0.02 | 0.64 ± 0.02 | 0.68 ± 0.02 ** | 0.58 ± 0.02 *** |
| 7 | 0.43 ± 0.03 | 0.68 ± 0.03 | 0.72 ± 0.03 | 0.60 ± 0.03 *** |
| 8 | 0.47 ± 0.01 | 0.65 ± 0.01 | 0.58 ± 0.01 *** | 0.52 ± 0.01 *** |
| 9 | 0.43 ± 0.01 | 0.59 ± 0.01 ** | 0.63 ± 0.01 *** | 0.66 ± 0.02 *** |
| 10 | 0.48 ± 0.03 | 0.61 ± 0.03 | 0.76 ± 0.03 | 0.64 ± 0.03 *** |
| 11 | 0.42 ± 0.01 | 0.66 ± 0.01 | 0.63 ± 0.01 *** | 0.57 ± 0.01 *** |
| Groups | % Inhibition of Acute Inflammation | ||
|---|---|---|---|
| 1 h | 2 h | 3 h | |
| Control | 0 | 0 | 0 |
| Indomethacin (10 mg/kg) | 8.45 | 26.83 | 33.33 |
| 1 | 7.04 | 12.20 | 23.81 |
| 2 | 18.31 | 17.07 | 27.38 |
| 3 | 21.13 | 19.51 | 32.14 |
| 4 | 29.58 | 30.49 | 44.05 |
| 5 | 11.27 | 14.64 | 28.57 |
| 6 | 9.86 | 17.07 | 30.95 |
| 7 | 4.23 | 12.20 | 28.57 |
| 8 | 8.45 | 29.27 | 38.10 |
| 9 | 16.90 | 23.17 | 21.43 |
| 10 | 14.08 | 7.32 | 23.81 |
| 11 | 7.04 | 23.17 | 32.14 |

2.3. Molecular Docking Study
| Compound No. | Chemical Structure | dG (COX-1) Kcal/mole | dG(COX-2) Kcal/mole |
|---|---|---|---|
| Reference |  | −11.5716 | −11.1006 |
| 1 |  | −12.2721 | −10.5499 |
| 2 |  | −11.5564 | −10.7125 |
| 3 | 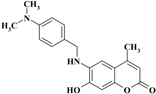 | −11.1199 | −10.9148 |
| 4 |  | −13.5408 | −11.2977 |
| 5 | 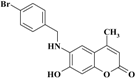 | −11.3127 | −11.1787 |
| 6 | 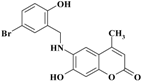 | −11.5776 | −10.7572 |
| 7 | 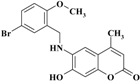 | −11.0632 | −10.9992 |
| 8 |  | −12.4821 | −12.2774 |
| 9 |  | −11.3067 | −10.4561 |
| 10 |  | −11.7976 | −11.2097 |
| 11 |  | −11.3457 | −10.8248 |
2.3.1. Binding Modes of Different Compounds with COX-2 Enzyme





2.3.2. Binding Modes of Different Compounds with the COX-1 Enzyme





3. Experimental Section
3.1. General Information
3.1.1. 7-Hydroxy-4-methyl-2H-chromen-2-one (III)
3.1.2. 7-Hydroxy-4-methyl-6-nitro-2H-chromen-2-one (IV)
3.1.3. 6-Amino-7-hydroxy-4-methyl-2H-chromen-2-one (V)
3.2. Anti-Inflammatory Screening
3.2.1. Carrageenan-Induced Paw Edema Method
3.2.2. Statistical Analysis
3.3. Molecular Docking
3.3.1. Target Compounds Optimization
3.3.2. Optimization of the Enzymes Active Site
3.3.3. Docking of the Target Molecules to the COX-1 and COX-2 Active Sites
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurumbail, G.R.; Stevens, M.A.; Gierse, K.J.; Mc Donald, J.J.; Stegeman, A.R.; Pak, Y.J.; Gildehaus, D.; Miyashiro, M.J.; Pennung, D.T. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.S.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Huang, H.C.; Lee, C.R.; Weng, Y.I.; Lee, M.; Lee, Y.T. Vasodilator effect of Scoparone (6,7-dimethoxycoumarin) from a Chinese herb. Eur. J. Pharmacol. 1992, 218, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Buckle, D.R.; Outred, D.J.; Ross, J.W.; Smith, H.; Smith, R.J.; Spicer, B.A.; Gasson, B.C. Aryloxyalkyloxy and aralkyloxy-4-hydroxy-3-nitrocoumarins which inhibit histamine release in the rat and also antagonize the effects of a slow reacting substance of anaphylaxis. J. Med. Chem. 1979, 22, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with antiinflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological evaluation of several coumarin derivatives designed as possible antiinflammatory/antioxidant agents. J. Enzym. Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Hadjipavlou-Litina, D.J.; Kontogiorgis, C.A. Synthesis and biological evaluation of benzo [7,8] chromeno [5,6-b][1,4] oxazin-3-ones. J. Heterocycl. Chem. 2004, 41, 605–611. [Google Scholar] [CrossRef]
- Koh, M.S.; Parnete, L.; Willoughby, D.A. Immunological and non-immunological pleural inflammation: The effects of anti-inflammatory and anti-rheumatic drugs. Eur. J. Rheumatol. Inflamm. 1978, 1, 286–290. [Google Scholar]
- Hadjipavlou-Litina, D.J.; Litinas, K.E.; Kontogiorgis, C. The anti-inflammatory effect of coumarin and its derivatives. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2007, 6, 293–306. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced oedema in hind paw of rat as the assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 3, 544–547. [Google Scholar] [CrossRef]
- Kiefer, J.R.; Pawlitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Gierse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J.; et al. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.A.; Farag, N.A.; Soliman, G.A. Synthesis, biological evaluation and docking studies of novel benzopyranone congeners for their expected activity as anti-inflammatory, analgesic and antipyretic agents. Bioorg. Med. Chem. 2009, 17, 5059–5070. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, H.V.; Duisberg, C. Über die verbindungen der phenole mit acetessigäther. Ber. Dtsch. Chem. Ges. 1883, 16, 2119–2128. [Google Scholar] [CrossRef]
- Shah, N.M.; Mehta, D.H. Nitration of 7-hydroxy-4-methylcoumarin and its methyl ether. J. Ind. Chem. Soc. 1954, 31, 784–786. [Google Scholar]
- Kaufmann, K.D.; Russey, W.E.; Worden, L.R. Synthetic furocoumarins. V.1 preparation and reactions of 8-Amino-4,5'-dimethylpsoralene. J. Org. Chem. 1962, 27, 875–879. [Google Scholar] [CrossRef]
- Olfert, E.D.; Cross, B.M.; McWilliam, A.A. Guide to the Care and Use of Experimental Animals,, 2nd ed.; Bradda Printing Services Inc.: Ottawa, ON, Canada, 1993; Volume 1. [Google Scholar]
- Chattopadhyay, D.; Arunachalam, G.; Mandal, A.B.; Sur, T.K.; Mandal, S.C.; Bhattacharya, S.K. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J. Ethnopharmacol. 2002, 82, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), version 2010.10; Chemical Computing Group Inc.: Montreal, QC, Canada, 2013.
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Haggar, R.; Al-Wabli, R.I. Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives. Molecules 2015, 20, 5374-5391. https://doi.org/10.3390/molecules20045374
El-Haggar R, Al-Wabli RI. Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives. Molecules. 2015; 20(4):5374-5391. https://doi.org/10.3390/molecules20045374
Chicago/Turabian StyleEl-Haggar, Radwan, and Reem I. Al-Wabli. 2015. "Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives" Molecules 20, no. 4: 5374-5391. https://doi.org/10.3390/molecules20045374






