Studies on the Alkaloids of the Calycanthaceae and Their Syntheses
Abstract
:1. Introduction
2. Structures, Biological Activities, and Biosynthetic Origins of Calycanthaceae Alkaloids
2.1. Structures of Calycanthaceae Alkaloids and Their Discovery

2.2. Biological Activities
2.3. Biosynthetic Origins


3. Total Synthesis of Calycanthaceae Alkaloids
3.1. Calycanthines and Chimonanthines








3.2. Folicanthines

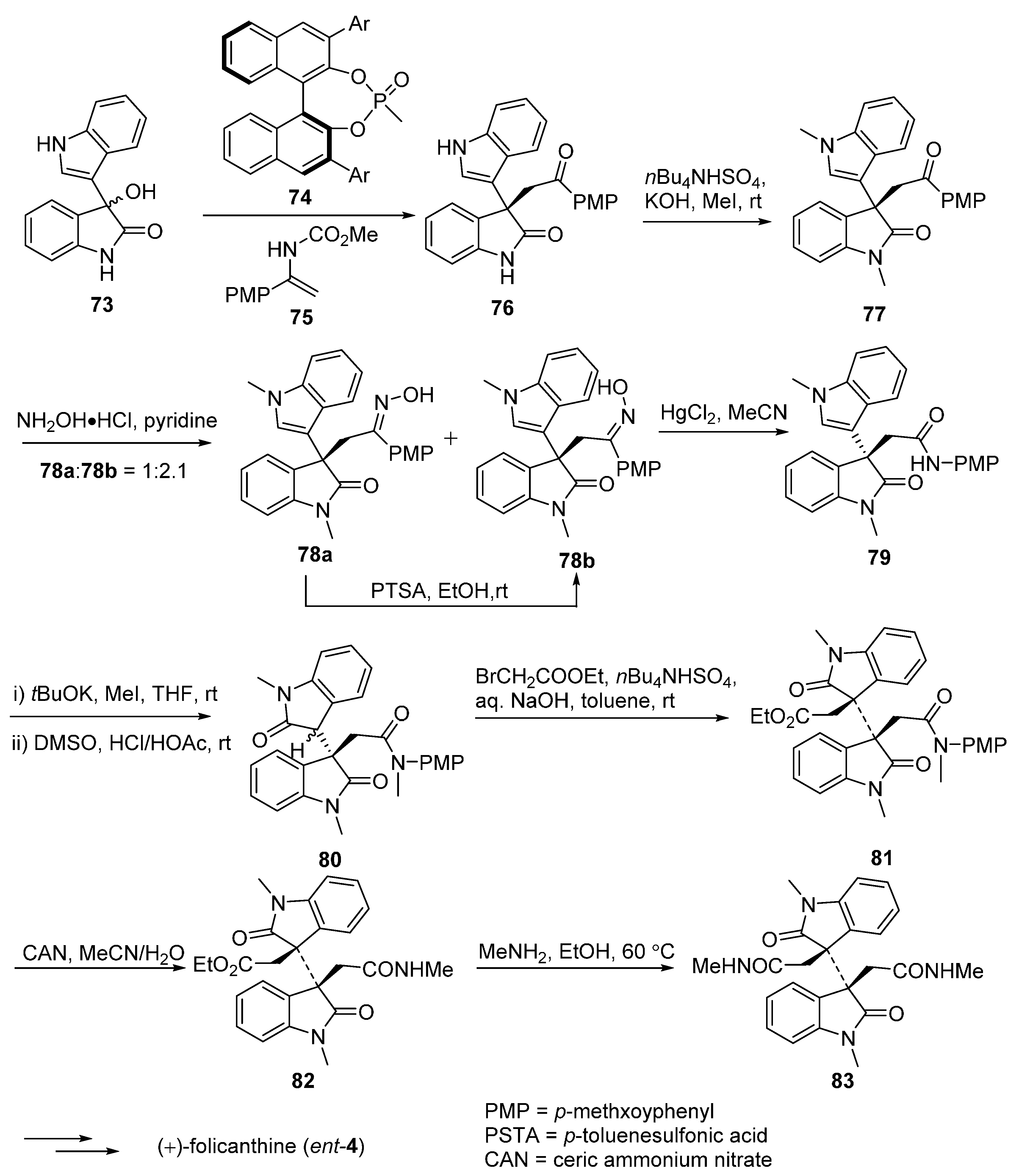


3.3. (−)-Idiospermuline (6)
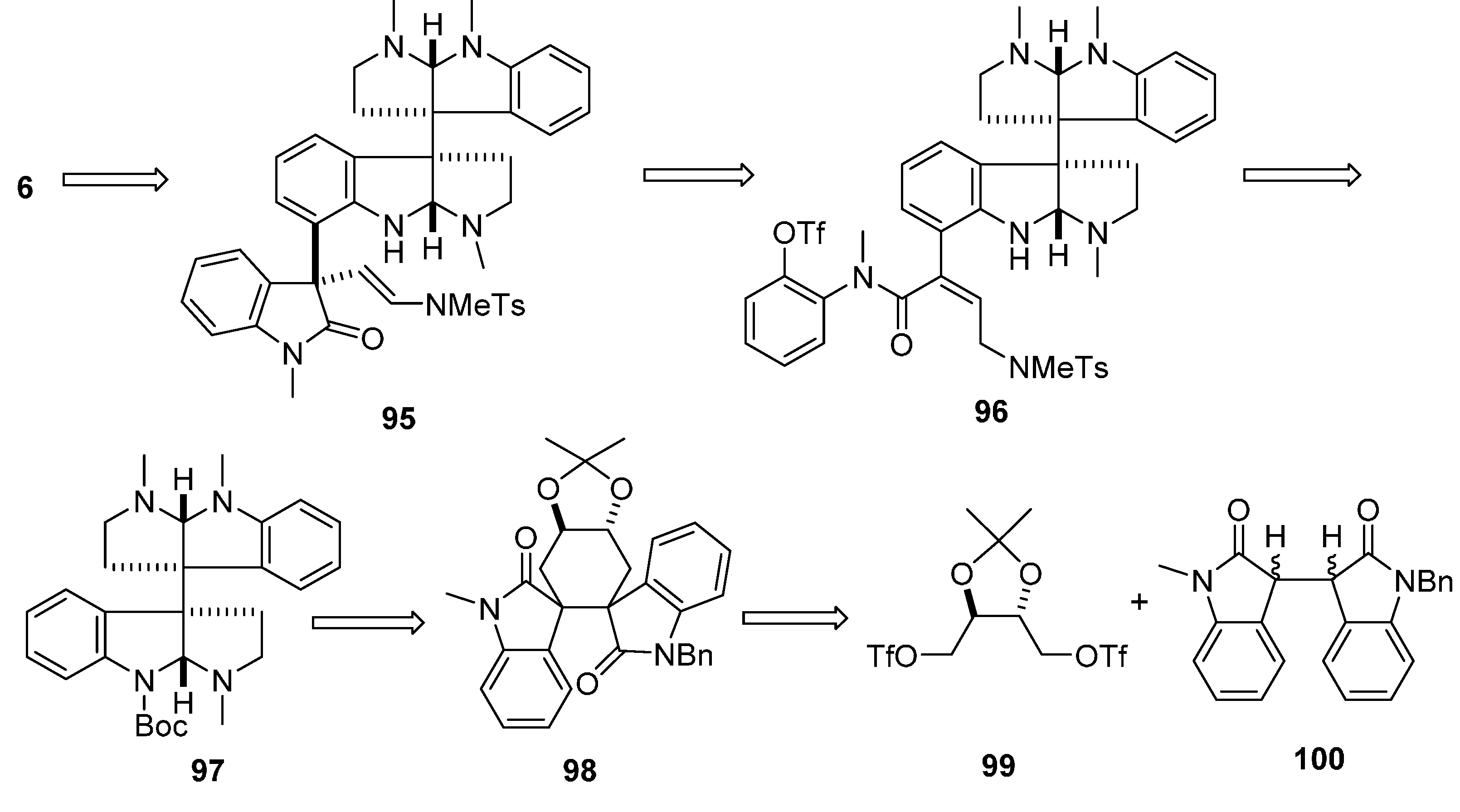

3.4. Chimonamidines (7)

3.5. Chimonanthidine (8)

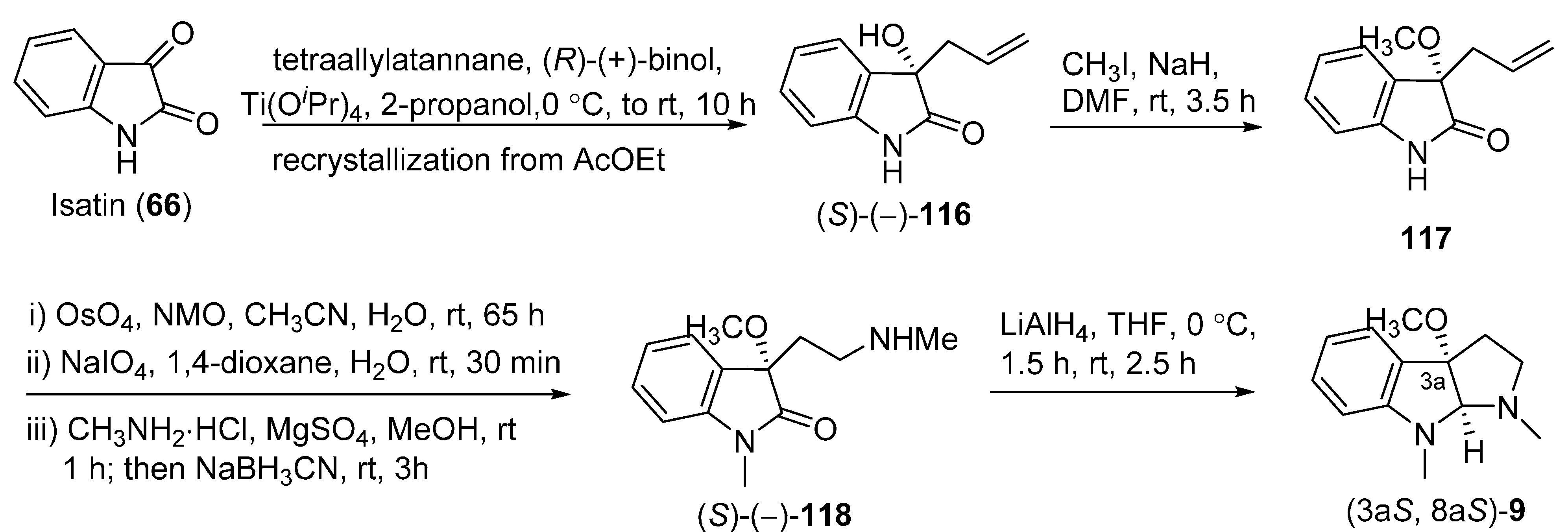
3.6. Rac-CPC-1 (Rac-9)

4. Conclusions
Acknowledgments
Author Contributions
List of Abbreviations
| Ac | acetyl |
| Bn | benzyl |
| Boc | tert-butoxycarbonyl |
| CAN | ceric ammonium nitrate |
| CAS | camphorsulfonic acid monohydrate |
| Cbz | carbobenzoxy |
| m-CPBA | m-cholorperoxybenzoic acid |
| dba | trans,trans-dibenzylideneacetone |
| dppe | 1,2-bis(diphenylphosphino)ethane |
| dppb | bis(1,4-diphenylphosphanyl)butane |
| DMA | N,N-dimethylacetamide |
| DMF | 2,5-dimethylfuran |
| DMSO | dimethyl sulfoxide |
| 2,2-DMP | 2,2-dimethioxypropane |
| DMPU | 1,3-dimethylhexahydro-2-pyrimidinone |
| HMDS | 1,1,1,3,3,3-hexamethyldisilazane |
| HMPA | hexamethylphoramide |
| NaHMDS | sodium hexamethyldisilazide |
| NMO | 4-methylmorpholine-N-oxide |
| PIFA | phenyliodine(III) bis(trifluoroacetate) |
| PMP | 1,2,2,6,6-pentamethylpiperidine |
| PSTA | p-toluenesulfonic acid |
| Red-Al | sodium bis(2-methoxyethoxy)aluminum hydride |
| TBAF | tertrabutylammonium fluoride |
| Tf | trifluoromethanesulfonyl |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TMEDA | N,N,N,N-tetramethylethylenediamine |
| TMS | trimethylsilyl |
Conflicts of Interest
References
- Li, Y.; Li, B.T. Cladistic analysis of calycanthaceae. J. Trop. Subtrop. 2000, 8, 275–281. [Google Scholar]
- Zhou, S.L.; Renner, S.S.; Wen, J. Molecular phylogeny and intra- and intercontinental biogeography of Calycanthaceae. Mol. Phylogenetics Evol. 2006, 39, 1–15. [Google Scholar]
- Chen, L.Q. Research advances on Calycanthaceae. Chin. Landsc. Archit. 2012, 8, 49–53. [Google Scholar]
- Dai, P.F.; Yang, J.; Zhou, T.H.; Huang, Z.H.; Feng, L.; Su, H.L.; Liu, Z.L.; Zhao, G.F. Genetic diversity and differentiation in Chimonanthus praecox and Ch. salicifolius (Calycanthaceae) as revealed by inter-simple sequence repeat (ISSR) markers. Biochem. Syst. Ecol. 2012, 44, 149–156. [Google Scholar]
- Jiang, Y.; Li, B.T.; Li, Y.H. Chinese Flora (Zhongguo Zhiwu Zhi); Science Press: Beijing, China, 1979; Volume 30, p. 5. [Google Scholar]
- Wang, L.Y.; Zhang, Z.B.; Zou, Z.R.; Zhu, D. Advances of studies on chemical composition and pharmacological activity of Chimonanthus Lindl. LiShiZhen Medicaine Mater. Med. Res. 2012, 23, 3103–3106. [Google Scholar]
- Lv, J.S.; Zhang, L.L.; Chu, X.Z.; Zhou, J.F. Chemical composition, antioxidant and antimicrobial activity of the extracts of the flowers of the Chinese plant Chimonanthus praecox. Nat. Prod. Res. 2012, 26, 1363–1367. [Google Scholar]
- Liu, Z.Z.; Xi, J.; Schröder, S.; Wang, W.G.; Xie, T.P.; Wang, Z.G.; Bao, S.S.; Fei, J. Chimonanthus nitens var. salicifolius aqueous extract protects against 5-fluorouracil induced gastrointerstinal mucostis in a mouse model. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Xiao, P.G. Modern Chinese Materia Medica; Chemical Industry Press: Beijing, China, 2001; Volume 3, p. 388. [Google Scholar]
- Sun, L.R.; He, M.Z.; Feng, Y.L.; Chen, K.P.; Jian, H.; Wang, Y.S.; Yang, S.L. Studies on the chemical constituents of Chimonanthus nitens. Chin. Tradit. Hreb. Drugs 2009, 40, 1214–1216. [Google Scholar]
- Shu, R.G.; Li, S.S.; Hu, H.W.; Zhang, P.Z. Studies on chemical constituents of Chimonanthus nitens Oliv. Chin. Pharm. J. 2010, 45, 1134–1135. [Google Scholar]
- Li, Q.J.; Wang, M.L.; Yang, X.S.; Ma, L.; Hao, X.J. Two new coumarins glycosides from Chimonanthus nitens. J. Asian Nat. Prod. Res. 2013, 15, 270–275. [Google Scholar]
- Zhang, Y.; Hua, J.W.; Wang, X.Y.; Cheng, W.L.; Lei, H.X.; Cheng, K.J.; Yu, P.Z. Chemical constituents of chloroform fraction from leaves of Chimonanthus salicifolius. Zhongguo Zhongyao zazhi (China J. Chin. Matera Med.) 2013, 38, 2661–2664. [Google Scholar]
- Xiao, B.K.; Liu, Y.M.; Feng, S.X.; Huang, R.Q.; Luo, C.H.; Dong, J.X. Studies on the chemical constituents of Chimonanthus nitens Oliv (I). Chin. Tradit. Herb. Drugs 2005, 36, 187–189. [Google Scholar]
- Wang, W.X.; Cao, L.; Xiong, J.; Xia, G.; Hu, J.F. Constituents from Chimonanthus praecox (wintersweet). Phytochem. Lett. 2011, 4, 271–274. [Google Scholar]
- Kashiwada, Y.; Nishimura, K.; Kurimoto, S.; Takaishi, Y. New 29-nor-cycloartanes with a 3,4-seco- and a novel 2,3-seco-structure from the leaves of Sinocalycanthus chinensis. Bioorg. Med. Chem. 2011, 19, 2790–2796. [Google Scholar]
- Collins, R.P.; Halim, A.F. Essential leaf oils in Calycanthus floridus. Planta Med. 1971, 20, 241–243. [Google Scholar]
- Yu, C.L.; Kuang, Y.; Yang, S.X.; Liu, L.; Liu, C.G. Chemical composition, antifungal activity and toxicity of essential oils from leaves of Chimonanthus praecox and Chimonanthus zhejianggensis. Asian J. Chem. 2014, 26, 254–256. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Wang, Z.Z. Chemical composition and biological activities of essential oil from flower of Chimonanthus praecox (L.) Link. LiShiZhen Med. Mater. Med. Res. 2010, 21, 622–625. [Google Scholar]
- Deng, C.; Song, G.; Hu, Y. Rapid determination of volatile compounds emitted from Chimonanthus praecox flowers by HS-SPME-GC-MS. Z. Naturforschung C 2004, 59, 636–640. [Google Scholar]
- Gordin, H.M. On the crystalline alkaloid of Calycanthus glaucus. J. Am. Chem. Soc. 1905, 27, 144–155. [Google Scholar]
- Gordin, H.M. On the crystalline alkaloid of Calycanthus glaucus. Second paper. J. Am. Chem. Soc. 1905, 27, 1418–1429. [Google Scholar]
- Späth, E.; Stroh, W. Zur Kenntnis des Caly-canthins. Ber. Dtsch. Chem. Ges. 1925, 58, 2131–2132. [Google Scholar]
- Manske, R.H.F. Calycanthine, I. The isolation of calycanthine from Meratia praecox. J. Am. Chem. Soc. 1929, 51, 1836–1839. [Google Scholar]
- Barger, G.; Madinaveitia, J.; Streuli, P. Calycanthine. J. Chem. Soc. 1939, 510–517. [Google Scholar]
- Hamor, T.A.; Robertson, J.M.; Shrivastava, H.N.; Silverton, J.V. The structure of calycanthine. Proc. Chem. Soc. 1960, 78–80. [Google Scholar]
- Gordin, H.M. On the crystalline alkaloid of Calycanthus glaucus. Third paper. On isocalycanthine, isomeric with calycanthine. J. Am. Chem. Soc. 1905, 31, 1305–1312. [Google Scholar]
- Gordin, H.M. The crystalline alkaloid of Calycanthus glaucus. Fourth paper. Some salts of a new quaternary base obtained by methylating isocalycanthine. J. Am. Chem. Soc. 1911, 33, 1626–1632. [Google Scholar]
- Adjibade, Y.; Weniger, B.; Quirion, J.C.; Kuballa, B.; Cabaline, P.; Anton, R. Dimeric alkaloids from Psychotria forsteriana. Phytochemistry 1992, 31, 317–319. [Google Scholar]
- Barger, G.; Jacob, A.; Madinaveitia, J. Calycanthidine, a new simple indole alkaloid. Recl. Trav. Chim. Pays-Bas 1938, 57, 548–554. [Google Scholar]
- Saxton, J.E.; Bardsley, W.G.; Smith, G.F. The structure of calycanthidine. Proc. Chem. Soc. 1962, 148. [Google Scholar]
- Hodson, H.F.; Robinson, B.; Smith, G.F. Chimonanthine, a new calycanthaceous alkaloid. Proc. Chem. Soc. 1961, 465–466. [Google Scholar]
- Grant, I.J.; Hamor, T.A.; Robertson, J.M.; Sim, G.A. The structure of chimonanthine. Proc. Chem. Soc. 1962, 148–149. [Google Scholar]
- Grant, I.J.; Hamor, T.A.; Robertson, J.M.; Sim, G.A. The structure of Chimonanthine: X-ray ananlysis of Chimonanthine dihydrobromide. J. Chem. Soc. 1965, 5678–5696. [Google Scholar]
- Overman, L.E.; Larrow, J.F.; Steaens, B.A.; Vance, J.M. Enantioselective construction of vicinal stereogenic quaternary centers by dialkylation: practical total syntheses of (+)- and meso-chimonanthine. Angew. Chem. Int. Ed. 2000, 39, 213–215. [Google Scholar]
- Woodward, R.B.; Yang, N.C.; Katz, T.J.; Clark, V.M.; Harley-Mason, J.; Ingleby, R.F.J.; Sheppard, N. Calycanthine: The structure of the alkaloid and its degradation product, calycanine. Proc. Chem. Soc. 1960, 76–78. [Google Scholar]
- Duke, R.K.; Allan, R.D.; Johnston, G.A.R.; Mewett, K.N.; Mitrovic, A.D. Idiospermuline, a trimeric pyrrolidinoindoline alkaloid from the seed of Idiospermum australiense. J. Nat. Prod. 1995, 58, 1200–1208. [Google Scholar]
- Takayama, H.; Matsuda, Y.; Masubuchi, K.; Ishida, A.; Kitajima, M.; Aimi, N. Isolation, structure elucidation, and total synthesis of two new Chimonanthus alkaloids, chimonamidine and chimonanthidine. Tetrahedron 2004, 60, 893–900. [Google Scholar]
- Kitajima, M.; Mori, I.; Arai, K.; Kogure, N.; Takayama, H. Two new tryptamine-derived alkaloids from Chimonanthus praecox f. concolor. Tetrahedron Lett. 2006, 47, 3199–3202. [Google Scholar]
- Zhang, J.W.; Gao, J.M.; Xu, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014, 68, 539–549. [Google Scholar]
- Chen, A.L.; Powell, C.E.; Chen, K.K. The action of calycanthine. J. Am. Pharm. Assoc. 1942, 31, 513–516. [Google Scholar]
- Adhibadé, Y.; Hue, B.; Pelhate, M.; Anton, R. Action of calycanthine on nervous transmission in cockroach central nervous system. Planta Med. 1991, 57, 99–101. [Google Scholar]
- Chebib, M.; Duke, R.K.; Duke, C.C.; Connor, M.; Mewett, K.N.; Johnston, G.A.R. Convulsant actions of calycanthine. Toxicol. Appl. Pharmacol. 2003, 190, 58–64. [Google Scholar]
- Shi, L.J.; Yang, S.X.; Bi, J.L.; Yin, G.F.; Wang, Y.H. Chemical constituents from the branches and leaves of Chimonanthus praecox with antiviral activity investigations. Nat. Prod. Res. Dev. 2012, 24, 1335–1338. [Google Scholar]
- Amador, T.A.; Verotta, L.; Nunes, D.S.; Elisabetsky, E. Antinociceptive profile of hodgkinsine. Planta Med. 2000, 66, 770–772. [Google Scholar]
- Verotta, L.; Orsini, F.; Sbacchi, M.; Scheildler, M.A.; Amador, T.A.; Elisabetsky, E. Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids. Bioorg. Med. Chem. 2002, 10, 2133–2142. [Google Scholar]
- May, J.A.; Stoltz, B. The structural and synthetic implications of the biosynthesis of the calycanthaceous alkaloids, the communesins, and nomofungin. Tetrahedron 2006, 62, 5262–5271. [Google Scholar]
- Hino, T. Synthetic approaches to Calycanthuaceae alkaloids. II. A synthesis of 1,1'-dimethyl-3,3'-bis(2-aminoethyl)-3,3'-bioxindole. Chem. Pharm. Bull. 1961, 9, 979–988. [Google Scholar]
- Hino, T.; Yamada, S. Total synthesis of (±)-folicanthine. Tetradedron. Lett. 1963, 4, 1757–1760. [Google Scholar]
- Hall, E.S.; McCapra, F.; Scott, A.I. Biogenetic-type synthesis of the calycanthaceous alkaloids. Tetrahedron 1967, 23, 4131–4141. [Google Scholar]
- Hendrickson, J.B.; Göschke, R.; Rees, R. Total synthesis of calycanthaceous alkaloids. Tetrahedron 1964, 20, 565–579. [Google Scholar]
- Fang, C.L.; Horne, S.; Taylor, N.; Rodrigo, R. Dimerization of a 3-substituted oxindole at C-3 and its application to the synthesis of (±)-folicanthine. J. Am. Chem. Soc. 1994, 116, 9480–9486. [Google Scholar]
- Scott, A.I.; McCapra, F.; Hall, E.S. Chimonanthine, a one-step synthesis and biosynthetic model. J. Am. Chem. Soc. 1964, 86, 302–303. [Google Scholar]
- Link, J.T.; Overman, L.E. Stereocontrolled total syntheses of meso-chimonanthine and meso-calycanthine via a novel samarium mediated reductive dialkylation. J. Am. Chem. Soc. 1996, 118, 8166–8167. [Google Scholar]
- Overman, L.E.; Paone, D.V.; Stearns, B.A. Direct stereo- and enantiocontrolled synthesis of vicinal stereogenic quaternary carbon centers. Total syntheses of meso- and (−)-chimonanthine and (+)-calycanthine. J. Am. Chem. Soc. 1999, 121, 7702–7703. [Google Scholar]
- Menozzi, C.; Dalko, P.I.; Cossy, J. Concise synthesis of the Nb-desmethyl-meso-chimonanthine. Chem. Commun. 2006, 4638–4640. [Google Scholar]
- Movassaghi, M.; Schmidt, M.A. Concise total synthesis of (−)-calycanthine, (+)-chimonanthine, and (+)-folicanthine. Angew. Chem. Int. Ed. 2007, 46, 3725–3728. [Google Scholar]
- Lathrop, S.P.; Movassaghi, M. Application of diazene-directed fragment assembly to the total synthesis and stereochemical assignment of (+)-desmethyl-meso-chimonanthine and related heterodimeric alkaloids. Chem. Sci. 2014, 5, 333–340. [Google Scholar]
- Movassaghi, M.; Ahmad, O.K.; Lathrop, S.P. Directed heterodimerization: Stereocontrolled assembly via solvent-caged unsymmetrical diazene fragmentation. J. Am. Chem. Soc. 2011, 133, 13002–13005. [Google Scholar]
- Ellis, J.M.; Overman, L.E.; Tanner, H.R.; Wang, J. A versatile synthesis of unsymmetical 3,3'-bioxindoles: Stereoselective Mukaiyama Aldol reactions of 2-siloxyindoles with isatins. J. Org. Chem. 2008, 73, 9151–9154. [Google Scholar]
- Mitsunuma, H.; Shibasaki, M.; Kanai, M.; Matsunaga, S. Catalytic asymmetric total synthesis of chimonanthine, folicanthine, and calycanthine through double Michael reaction of bisoxindole. Angew. Chem. Int. Ed. 2012, 51, 5217–5221. [Google Scholar]
- Araki, T.; Manabe, Y.; Fujioka, K.; Yokoe, H.; Kanematsu, M.; Yoshida, M.; Shishido, K. Total syntheses of (±)-folicanthine and (±)-chimonanthine via a double intramolecular carbamoylketene-alkene [2 + 2] cycloaddition. Tetrahedron Lett. 2013, 54, 1012–1014. [Google Scholar]
- Guo, C.; Song, J.; Huang, J.Z.; Chen, P.H.; Luo, S.W.; Gong, L.Z. Core-structure-oriented asymmetric organocatalytic substitution of 3-hydroxyoxindoles: Application in the enantioselective total synthesis of (+)-folicanthine. Angew. Chem. Int. Ed. 2012, 51, 1046–1050. [Google Scholar]
- Li, Y.X.; Wang, H.X.; Ali, S.; Xia, X.F.; Liang, Y.M. Iodine-mediated regioselective C2-amination of indoles and a concise total synthesis of (±)-folicanthine. Chem. Commun. 2012, 48, 2343–2345. [Google Scholar]
- Kodanko, J.J.; Overman, L.E. Enantioselective total syntheses of the cyclotryptamine alkaloids hodgkinsine and hodgkinsine B. Angew. Chem. Int. Ed. 2003, 42, 2528–2531. [Google Scholar]
- Overman, L.E.; Peterson, E.A. Enantioselective total synthesis of the cyclotryptamine alkaloid idiospemuline. Angew. Chem. Int. Ed. 2003, 42, 2525–2528. [Google Scholar]
- Overman, L.E.; Peterson, E.A. Enantioselective synthesis of (−)-idiospemuline. Tetrahedron 2003, 59, 6905–6919. [Google Scholar]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2963. [Google Scholar]
- Somei, M.; Yamada, F. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat. Prod. Rep. 2005, 22, 73–103. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-B.; Cheng, K.-J. Studies on the Alkaloids of the Calycanthaceae and Their Syntheses. Molecules 2015, 20, 6715-6738. https://doi.org/10.3390/molecules20046715
Xu J-B, Cheng K-J. Studies on the Alkaloids of the Calycanthaceae and Their Syntheses. Molecules. 2015; 20(4):6715-6738. https://doi.org/10.3390/molecules20046715
Chicago/Turabian StyleXu, Jin-Biao, and Ke-Jun Cheng. 2015. "Studies on the Alkaloids of the Calycanthaceae and Their Syntheses" Molecules 20, no. 4: 6715-6738. https://doi.org/10.3390/molecules20046715




