Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Human Monoamine Oxidase (hMAO) Inhibitory Activities

| Compounds | ΔG for MAO-A | Calculated Ki for MAO-A(μM) | ΔG for MAO-B | Calculated Ki for MAO-B(μM) | Calculated SI ** | Experimental Ki for MAO-A * (μM) | Experimental Ki for MAO-B * (μM) | Experimental SI ** | Selectivity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −7.81 | 1.89 | −5.78 | 64.25 | 0.029 | 0.76 ± 0.03 | 99.54 ± 7.44 | 0.007 | MAO-A |
| 2 | −4.14 | 927.10 | +5.78 | - | - | 1100.50 ± 99.20 | 75,600.00 ± 912.50 | 0.015 | MAO-A |
| 3 | −3.79 | 1660.00 | +8.92 | - | - | 2800.45 ± 180.50 | 80,500.00 ± 950.00 | 0.034 | MAO-A |
| 4 | −8.27 | 0.867 | −7.42 | 3.62 | 0.239 | 0.54 ± 0.02 | 6.27 ± 0.36 | 0.086 | MAO-A |
| Selegiline | 14.90 ± 1.33 | 0.36 ± 0.02 | 41.39 | MAO-B | |||||
| Moclobemide | 0.014 ± 0.001 | 1.34 ± 0.03 | 0.010 | MAO-A |


| Compounds (0.50 μM) | hMAO-A Inhibition (%) | |
|---|---|---|
| Before Washing | After Washing | |
| 1 | 86.49 ± 5.21 | 9.23 ± 0.75 |
| 2 | 80.22 ± 5.66 | 10.90 ± 0.99 |
| 3 | 74.22 ± 4.89 | 13.35 ± 1.09 |
| 4 | 89.03 ± 5.02 | 8.66 ± 0.65 |
| Moclobemide | 91.22 ± 6.33 | 7.89 ± 0.52 |
2.2. Molecular Docking Studies
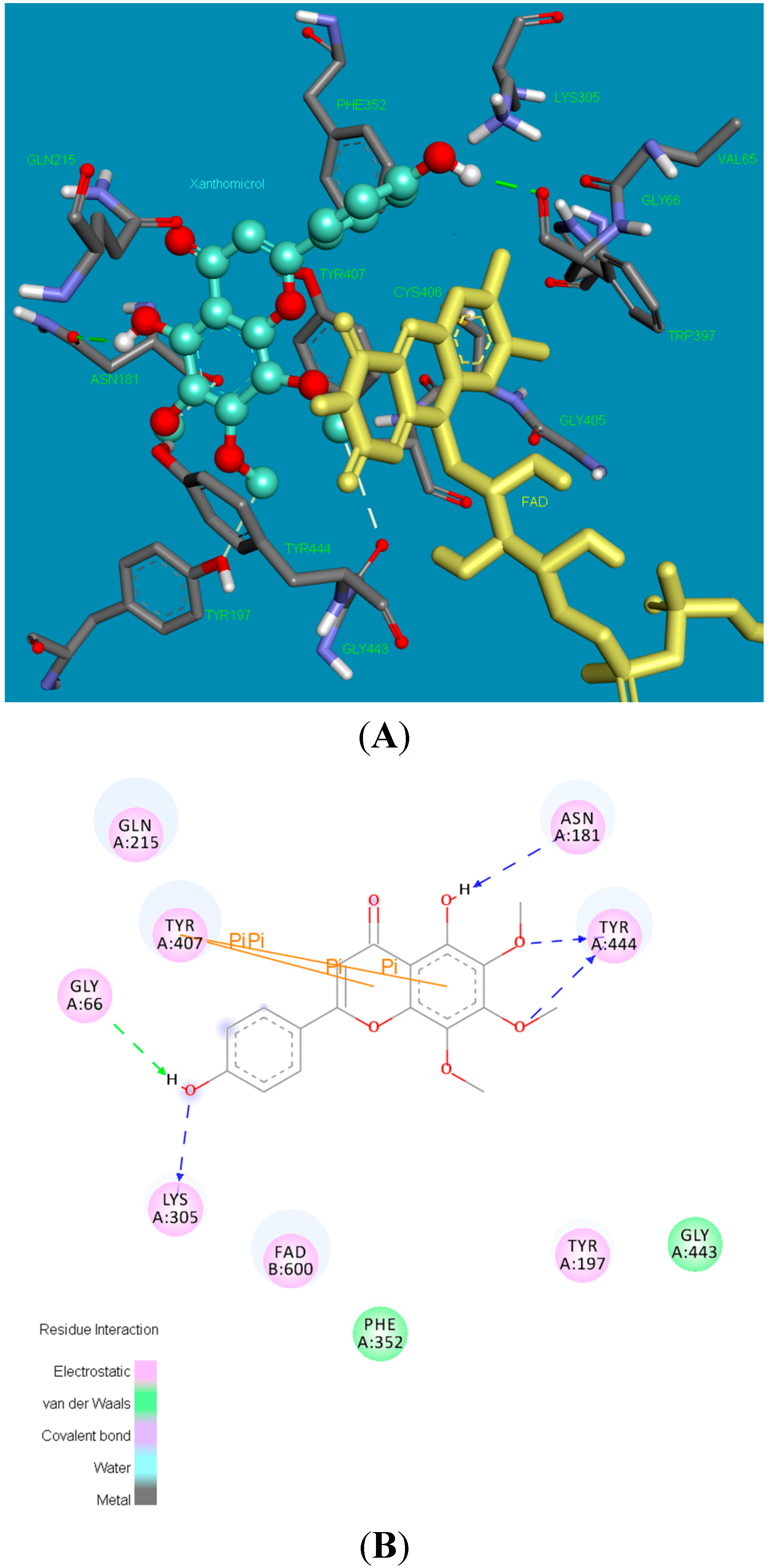



2.3. X-ray Crystallography

| Bond Angles (°) | Bond Angles (°) | Bond Angles (°) | |||
|---|---|---|---|---|---|
| C6 C1 C2 | 121.3(10) | C14 C13 O6 | 120.7(11) | O9 C21 H21B | 109.5 |
| C6 C1 H1 | 119.3 | C15 C14 O5 | 120.4(10) | H21A C21 H21B | 109.5 |
| C2 C1 H1 | 119.3 | C15 C14 C13 | 120.6(11) | O9 C21 H21C | 109.5 |
| O1 C2 C3 | 122.5(9) | O5 C14 C13 | 118.9(11) | H21A C21 H21C | 109.5 |
| O1 C2 C1 | 117.5(10) | O4 C15 C14 | 122.1(10) | H21B C21 H21C | 109.5 |
| C3 C2 C1 | 119.7(10) | O4 C15 C10 | 118.9(10) | O8 C22 C27 | 120.8(10) |
| C2 C3 C4 | 120.8(10) | C14 C15 C10 | 118.9(10) | O8 C22 C23 | 118.6(10) |
| C2 C3 H3 | 119.6 | O5 C16 H16A | 109.5 | C27 C22 C23 | 120.3(10) |
| C4 C3 H3 | 119.6 | O5 C16 H16B | 109.5 | C24 C23 C22 | 120.0(10) |
| C5 C4 C3 | 118.6(11) | H16A C16 H16B | 109.5 | C24 C23 O9 | 119.8(10) |
| C5 C4 H4 | 120.7 | O5 C16 H16C | 109.5 | C22 C23 O9 | 120.1(10) |
| C3 C4 H4 | 120.7 | H16A C16 H16C | 109.5 | O13 C24 C23 | 118.6(10) |
| C4 C5 C6 | 121.5(11) | H16B C16 H16C | 109.5 | O13 C24 C25 | 120.1(11) |
| C4 C5 C7 | 120.2(10) | O6 C17 H17A | 109.5 | C23 C24 C25 | 121.2(11) |
| C6 C5 C7 | 118.3(10) | O6 C17 H17B | 109.5 | C24 C25 C26 | 117.7(10) |
| C1 C6 C5 | 118.0(10) | H17A C17 H17B | 109.5 | C24 C25 C28 | 118.2(11) |
| C1 C6 H6 | 121.0 | O6 C17 H17C | 109.5 | C26 C25 C28 | 124.0(10) |
| C5 C6 H6 | 121.0 | H17A C17 H17C | 109.5 | O11 C26 C27 | 119.4(10) |
| C8 C7 O2 | 121.5(11) | H17B C17 H17C | 109.5 | O11 C26 C25 | 120.2(10) |
| C8 C7 C5 | 128.1(11) | O7 C18 H18A | 109.5 | C27 C26 C25 | 120.4(9) |
| O2 C7 C5 | 110.3(9) | O7 C18 H18B | 109.5 | O10 C27 C26 | 121.9(9) |
| C7 C8 C9 | 121.3(11) | H18A C18 H18B | 109.5 | O10 C27 C22 | 117.8(10) |
| C7 C8 H8 | 119.4 | O7 C18 H18C | 109.5 | C26 C27 C22 | 120.2(10) |
| C9 C8 H8 | 119.4 | H18A C18 H18C | 109.5 | O12 C28 C29 | 122.7(10) |
| O3 C9 C8 | 122.3(12) | H18B C18 H18C | 109.5 | O12 C28 C25 | 119.3(11) |
| O3 C9 C10 | 120.1(11) | O10 C19 H19A | 109.5 | C28 C29 H29 | 119.0 (9) |
| C8 C9 C10 | 117.4(9) | O10 C19 H19B | 109.5 | C29 C28 C25 | 118.0(10) |
| C11 C10 C15 | 119.6(10) | H19A C19 H19B | 109.5 | C29 C30 O13 | 120.0(10) |
| C11 C10 C9 | 117.6(10) | O10 C19 H19C | 109.5 | C29 C30 C31 | 128.0(8) |
| C15 C10 C9 | 122.8(10) | H19A C19 H19C | 109.5 | C30 C29 C28 | 122.0(10) |
| C10 C11 O2 | 122.6(10) | H19B C19 H19C | 109.5 | C30 C29 H29 | 119.0 |
| C10 C11 C12 | 121.7(10) | O8 C20 H20A | 109.5 | O13 C30 C31 | 111.8(10) |
| O2 C11 C12 | 115.6(9) | O8 C20 H20B | 109.5 | C36 C31 C32 | 114.9(9) |
| O7 C12 C13 | 122.0(10) | H20A C20 H20B | 109.5 | C36 C31 C30 | 122.4(10) |
| O7 C12 C11 | 120.5(10) | O8 C20 H20C | 109.5 | C32 C31 C30 | 122.5(10) |
| C13 C12 C11 | 117.2(9) | H20A C20 H20C | 109.5 | C33 C32 C31 | 122.3(10) |
| Bond Distances (Å) | Bond Distances (Å) | Bond Distances (Å) | |||
| C1 H1 | 0.93 1 | C15 O4 | 1.35(1) 1 | C22 C27 | 1.39(2) |
| C1 C2 | 1.39 (1) | C16 H16A | 0.96 1 | C22 O8 | 1.34(1) 1 |
| C1 C6 | 1.37(2) | C16 H16B | 0.96 1 | C23 C24 | 1.36(2) |
| C2 C3 | 1.36(2) | C16 H16C | 0.96 1 | C23 O9 | 1.42(1) 1 |
| C2 O1 | 1.36(1) 1 | C16 O5 | 1.42(1) 1 | C24 C25 | 1.42(2) |
| C3 H3 | 0.93 1 | C17 H17A | 0.96 1 | C24 O13 | 1.35(1) 1 |
| C3 C4 | 1.41(1) | C17 H17B | 0.96 1 | C25 C26 | 1.43(2) |
| C4 H4 | 0.93 1 | C17 H17C | 0.96 1 | C25 C28 | 1.43(2) |
| C4 C5 | 1.36(1) | C17 O6 | 1.38(2) 1 | C26 C27 | 1.38(2) |
| C5 C6 | 1.42(2) | C18 H18B | 0.96 1 | C26 O11 | 1.36(1) 1 |
| C5 C7 | 1.49(2) | C18 H18C | 0.96 1 | C27 O10 | 1.37(1) 1 |
| C6 H6 | 0.93 1 | C18 O7 | 1.38(1) 1 | C28 C29 | 1.40(2) |
| C7 C8 | 1.35(2) | O1 H1A | 0.821 1 | C28 O12 | 1.27(1) 1 |
| C7 O2 | 1.37(1) 1 | O3 H3A | 0.820 1 | C29 H29 | 0.93 1 |
| C8 H8 | 0.93 1 | O4 H4A | 0.819 1 | C29 C30 | 1.34(1) |
| C8 C9 | 1.41(2) | C19 H19A | 0.96 1 | C30 C31 | 1.45(1) |
| C9 C10 | 1.47(2) | C19 H19B | 0.96 1 | C30 O13 | 1.38(1) 1 |
| C9 O3 | 1.28(1) 1 | C19 H19C | 0.96 1 | C31 C32 | 1.43(1) |
| C10 C11 | 1.37(1) | C19 O10 | 1.35(1) 1 | C31 C36 | 1.36(2) |
| C10 C15 | 1.40(2) | H20A | 0.96 1 | C32 H32 | 0.93 1 |
| C11 C12 | 1.40(2) | H20B | 0.96 1 | C32 C33 | 1.34(1) |
| C11 O2 | 1.38(1) 1 | H20C | 0.96 1 | C33 H33 | 0.931 1 |
| C12 C13 | 1.37(2) | C20 O8 | 1.41(1) 1 | C33 C34 | 1.37(1) |
| C12 O7 | 1.34(1) 1 | C21 H21A | 0.96 1 | C34 C35 | 1.38(2) |
| C13 C14 | 1.39(2) | C21 H21C | 0.96 1 | C34 O14 | 1.37(1) 1 |
| C13 O6 | 1.39(1) 1 | C21 H21B | 0.96 1 | C35 H35 | 0.93 1 |
| C14 C15 | 1.37(2) | C21 O9 | 1.45(1) 1 | C35 C36 | 1.37(2) |
| C14 O5 | 1.38(1) 1 | C22 C23 | 1.40(2) | C36 H36 | 0.93 1 |
| O11 H11 | 0.820 1 | O12 H12 | 0.822 1 | O14 H14 | 0.823 1 |

| Parameter | Value |
|---|---|
| Empirical formula | C18H16O7 |
| M | 344 |
| Crystal system | Orthorhombic |
| Space group | Pna21 |
| a (Å) | 18.8736(14) |
| b (Å) | 4.1108(3) |
| c (Å) | 40.448(3) |
| α (°) | 90.00 |
| β (°) | 90.00 |
| γ (°) | 90.00 |
| Volume (Å3) | 3138.2(4) |
| Z | 8 |
| Dc/g·cm−3 | 1.389 |
| Absorption Coefficient (Mo Kα) mm−1 | 0.111 |
| Crystal size (mm) | 0.04 × 0.10 × 0.45 |
| Theta range for data collection (θ Range/°) | 2.014° to 25.344° |
| Index Ranges | −15 ≤ h ≤ 15, −3 ≤ k ≤ 3, −32 ≤ l ≤ 31 |
| Reflections collected | 3203 |
| Unique reflections, Rint | 1518 [R(int) = 0.0512] |
| Completeness of theta = 25.50° | 92% |
| Refinement Method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3203/1/463 |
| Goodness of fit on F2 | 1.030 |
| Final R indices [I > 2sigma(I)] | R1 = 0.1111, wR2 = 0.0584 I > 2σ(I) |
| R indices | R1 = 0.1460, wR2 = 0.1314 |
| Largest Diff. peak and hole (e·Å−3) | 0.210 and −0.198 e·Å−3 |
3. Experimental Section
3.1. Isolation of Flavonoids
3.2. Screening of hMAO Inhibitory Activities
3.2.1. Chemicals
3.2.2. Determination of hMAO-A and hMAO-B Activities
3.2.3. Kinetic Experiments
3.2.4. Reversibility Experiments
3.2.5. Molecular Docking Studies
3.3. X-ray Analysis
4. Conclusions
Supplementary Data
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obon de Castro, C.; Rivera-Nunez, D. Phanerogamarum Monographiae Tomus XXI: A Taxonomic Revision of the Section Sideritis (genus Sideritis) (Labiatae); J. Cramer: Berlin, Germany, 1994. [Google Scholar]
- Ezer, N.; Akcoş, Y. Flavonoids from Sideritis lycia. J. Hacettepe Univ. Fac. Pharm. 1995, 15, 81–87. [Google Scholar]
- Knörle, R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J. Neural Transm. 2012, 119, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities-a review. J. Ethnopharmacol. 1996, 135, 209–225. [Google Scholar]
- Barberan, F.A.T.; Tomas, F.; Ferreres, F. Isoscutellarein-7–0-allosyl (1–2) glucoside from Sideritis leucantha. J. Nat. Prod. 1985, 48, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Şahin, F.P.; Yeşilada, E.; Çalış, İ.; Ezer, N. In vivo anti-inflammatory and antinociceptive activity evaluation of phenolic compounds from Sideritis stricta. Z. Naturforschung 2007, 62c, 519–525. [Google Scholar]
- Baytop, T. Dağçayı. In Therapy with Medicinal plants in Turkey (Past and Present); Nobel Tip Publications: Istanbul, Turkey, 1999; p. 193. [Google Scholar]
- Ezer, N.; Vila, R.; Canigueral, S.; Adzet, T. Essential oil composition of four Turkish species of Sideritis. Phytochemistry 1996, 41, 203–205. [Google Scholar] [CrossRef]
- Baser, K.H.C. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure Appl. Chem. 2002, 74, 527–545. [Google Scholar] [CrossRef]
- Topçu, G.; Gören, A.C.; Kılıç, T.; Yıldız, Y.K.; Tümen, G. Diterpenes from Sideritis trojana. Nat. Prod. Lett. 2002, 16, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.P.; Ezer, N.; Çalış, İ. Three acylated flavone glycosides from Sideritis ozturkii Aytac & Aksoy. Phytochemistry 2004, 65, 2095–2099. [Google Scholar]
- Sahin, F.P.; Ezer, N.; Çalış, İ. Erratum to “three acylated flavone glycosides from Sideritis ozturkii Aytac and Aksoy” [Phytochemistry 65 (2004) 2095–2099]. Phytochemistry 2005, 66, 125. [Google Scholar]
- Sahin, F.P.; Ezer, N.; Calis, I. Terpenic and phenolic compounds from Sideritis stricta. Turk. J. Chem. 2006, 30, 495–504. [Google Scholar]
- Sezik, E.; Ezer, N. Phytochemical investigations on the plants used as folk medicine and herbal tea in Turkey I. Flavonoids of Sideritis congesta Davis et Huber-Morath. Acta Pharm. Turc. 1984, 16, 4–10. [Google Scholar]
- Ezer, N.; Sakar, M.K.; Rodriguez, B.; de la Torre, M.C. Flavonoid glycosides and a phenylpropanoid glycoside from Sideritis perfoliata. Int. J. Pharmacogn. 1992, 30, 61–65. [Google Scholar] [CrossRef]
- Güvenc, A.; Okada, Y.; Akkol, E.K.; Uman, H.; Okuyama, T.; Calis, I. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- Tipton, K.F.; Boyce, S.; O’Sullivan, J.; Davey, G.P.; Healy, J. Monoamine oxidases: Certainties and uncertainties. Curr. Med. Chem. 2004, 11, 1965–1982. [Google Scholar] [CrossRef] [PubMed]
- Westlund, K.N.; Denney, R.M.; Rose, R.M.; Abell, C.W. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 1988, 25, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.W.; Lan, N.C.; Johnson, D.L.; Abell, C.W.; Bembenek, M.E.; Kwan, S.W.; Seeburg, P.H.; Shih, J.C. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA 1988, 85, 4934–4938. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem. Pharmacol. 1968, 17, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Knoll, J.; Magyar, K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv. Biochem. Psychopharmacol. 1972, 5, 393–408. [Google Scholar] [PubMed]
- Pare, C.M. Unwanted effects of long-term medication in schizophrenia and depression. Pharmakop. Sych. Neuro. 1976, 9, 187–192. [Google Scholar]
- Shih, J.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Newton-Vinson, P.; Hubalek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002, 9, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Hubalek, F.; Li, M.; Edmondson, D.E.; Mattevi, A. Crystal structure of human monoamine oxidase B, a drug target enzyme monotopically inserted into the mitochondrial outer membrane. FEBS Lett. 2004, 564, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E.; Binda, C.; Mattevi, A. The FAD binding sites of human monoamine oxidases A and B. Neurotoxicology 2004, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAO A): Relation to the structures of rat MAO A and human MAO B. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Darvesh, A.S.; Funk, M.O.; Van der Schyf, C.J.; Carroll, R.T. Identification of novel monoamine oxidase B inhibitors by structure-based virtual screening. Bioorg. Med. Chem. Lett. 2010, 20, 5295–5298. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; D’Ascenzio, M.; Chimenti, P.; Secci, D.; Bolasco, A. Selective MAO-B inhibitors: A lesson from natural products. Mol. Divers. 2014, 18, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Suresh, J.; Mathew, G.E.; Parasuraman, R.; Abdulla, N. Plant secondary metabolites- potent inhibitors of monoamine oxidase isoforms. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fang, J.S.; Bai, X.Y.; Zhou, D.; Wang, Y.T.; Liu, A.L.; Du, G.H. In silico target fishing for the potential targets and molecular mechanisms of baicalein as an anti-parkinsonian agent: Discovery of the protective effects on NMDA receptor-mediated neurotoxicity. Chem. Biol. Drug Des. 2013, 81, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Sahin, F.P.; Calis, I.; Yesilada, E.; Ezer, N. Phenolic compounds of Sideritis ozturkii and their in vivo anti-inflammatory and antinociceptive activities. J. Ethnopharmacol. 2007, 112, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Tandogan, B.; Güvenç, A.; Çalış, İ.; Ulusu, N.N. In vitro effects of compounds isolated from Sideritis brevibracteata on bovine kidney cortex glutathione reductase. Acta Biochim. Pol. 2011, 58, 471–475. [Google Scholar]
- Youdim, M.B.H.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease. Br. J. Pharmacol. 2006, 147, S287–S296. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M.; Gillman, K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. In International Review of Neurobiology, Monoamine Oxidases and Their Inhibitors; Youdim, M.B.H., Riederer, P., Eds.; Academic Press: London, UK, 2011; Volume 100, pp. 169–190. [Google Scholar]
- Fowler, J.S.; Volkow, N.D.; Logan, J.; Wang, G.J.; MacGregor, R.R.; Schlyer, D.; Wolf, A.P.; Pappas, N.; Alexoff, D.; Shea, C.; et al. Slow recovery of human brain MAO B after l-deprenyl (seleginine) withdrawal. Synapse 1994, 18, 86–93. [Google Scholar]
- Lang, U.E.; Borgwardt, S. Molecular mechanisms of depression: Per-spectives on new treatment strategies. Cell Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Ginovart, N.; Boovariwala, A.; Sagrati, S.; Hussey, D.; Garcia, A.; Young, T.; Young, N.T.; Praschak-Rieder, N.; Wilson, A.A.; et al. Elevated monoamine oxidase A levels in the brain: An explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry 2006, 63, 1209–1216. [Google Scholar]
- Gong, J.; Huang, J.; Ge, Q.; Chen, F.; Zhang, Y. Advanced research on the antidepressant effect of flavonoids. Curr. Opin. Complement. Altern. Med. 2014, 1, e00011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Zheng, J.; Kwak, K.; Chen, X.; Asbury, J.B.; Fayer, M.D. Watching Ultrafast Molecular Motions with 2D IR Chemical Exchange Spectroscopy; World Scientific Publishing: London, UK, 2005. [Google Scholar]
- Kanko, C.; Koukoua, G.; N’Guessan, Y.T.; Fournier, J.; Pradère, J.P.; Toupet, L. Contribution à l’étude phytochimique de Lippiamultiflora (Verbenaceae). C. R. Chim. 2004, 7, 1029–1032. [Google Scholar] [CrossRef]
- Ouyang, X.; Wei, L.; Pan, Y.; Huang, S.; Wang, H.; Begonia, G.B.; Ekunwe, S.I.N. Antioxidant properties and chemical constituents of ethanolic extract and its fractions of Ocimum gratissimum. Med. Chem. Res. 2013, 22, 1124–1130. [Google Scholar] [CrossRef]
- Chimenti, F.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Carradori, S.; Alcaro, S.; Ortuso, F.; Yáñez, M. Synthesis, stereochemical identification, and selective inhibitory activity against human monoamine oxidase-B of 2-methylcyclohexylidene-(4-arylthiazol-2-yl) hydrazones. J. Med. Chem. 2008, 51, 4874–4880. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976, 72, 248–254. [Google Scholar]
- Chimenti, F.; Carradori, S.; Secci, D.; Bolasco, A.; Bizzarri, B.; Chimenti, P.; Granese, A.; Yáñez, M.; Orallo, F. Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di (hetero) aryl-4,5-dihydro-(1-H)-pyrazole derivatives. Eur. J. Med. Chem. 2010, 45, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Biological Macromolecular Resource. Available online: http://www.rcsb.org/pdb/home/ (accessed on 12 January 2015).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Yelekçi, K.; Büyüktürk, B.; Kayrak, N. In silico identification of novel and selective monoamine oxidase B inhibitors. J. Neural. Transm. 2013, 120, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gökhan-Kelekçi, N.; Şimşek, Ö.Ö.; Ercan, A.; Yelekçi, K.; Şahin, Z.S.; Işık, Ş.; Uçar, G.; Bilgin, A.A. Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorg. Med. Chem. 2009, 17, 6761–6772. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 4 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkmenoglu, F.P.; Baysal, İ.; Ciftci-Yabanoglu, S.; Yelekci, K.; Temel, H.; Paşa, S.; Ezer, N.; Çalış, İ.; Ucar, G. Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol. Molecules 2015, 20, 7454-7473. https://doi.org/10.3390/molecules20057454
Turkmenoglu FP, Baysal İ, Ciftci-Yabanoglu S, Yelekci K, Temel H, Paşa S, Ezer N, Çalış İ, Ucar G. Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol. Molecules. 2015; 20(5):7454-7473. https://doi.org/10.3390/molecules20057454
Chicago/Turabian StyleTurkmenoglu, Fatma Pinar, İpek Baysal, Samiye Ciftci-Yabanoglu, Kemal Yelekci, Hamdi Temel, Salih Paşa, Nurten Ezer, İhsan Çalış, and Gulberk Ucar. 2015. "Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol" Molecules 20, no. 5: 7454-7473. https://doi.org/10.3390/molecules20057454






