In Vivo Nanodetoxication for Acute Uranium Exposure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dendrimer Synthesis

| Full Name of Ligands | Ligands Molecular Structure |
|---|---|
| G4-Arg-Tos |  |
| G4-Lys-Fmoc-Cbz |  |
| G4-Lys-Cbz |  |
| G5-FA |  |
| G5-Cou |  |
| Dendrimer | Molecular Weight | Surface Group | n° Chemical Groups |
|---|---|---|---|
| G4 | 14,215 | Amine | 64 |
| G5 | 28,826 | Amine | 128 |
| G4-Arg-Tos | 19,800 | Arginine-Tos | 15 |
| G4-Lys-Fmoc-Cbz | 15,100 | Lysine-Fmoc-Cbz | 2 |
| G4-Lys-Cbz | 30,500 | Lysine-Cbz | 44 |
| G5-FA | 37,289 | Folic Acid | 18 |
| G5-Cou | 41,566 | Coumarine | 67 |
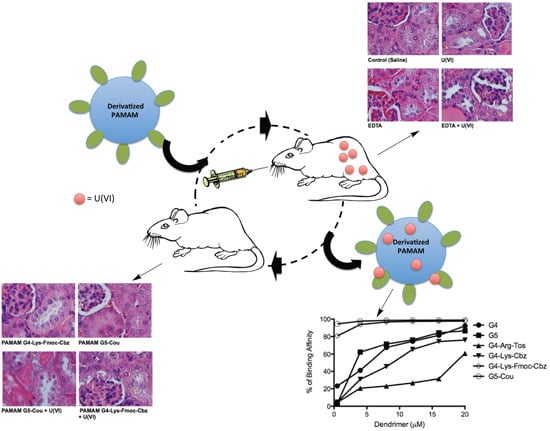
2.2. Effects of Derivatized PAMAM on U(VI) Trapping

| G4 | G5 | G4-Arg-Tos | G4-Lys-FMoc-Cbz | G4-Lys-Cbz | G5-FA | G5-Cou | |
|---|---|---|---|---|---|---|---|
| Cden | 5.0 × 10−7 | 5.0 × 10−7 | 5.0 × 10−7 | 5.0 × 10−7 | 5.0 × 10−7 | 5.0 × 10−7 | 5.0 × 10−7 |
| Ub | 8.7 × 10−6 | 4.0 × 10−6 | 2.0 × 10−7 | 1.6 × 10−5 | 2.0 × 10−7 | 2.0 × 10−7 | 1.9 × 10−5 |
| FBin (%) | 43.5 | 20.0 | 1.0 | 80.5 | 1.0 | 1.0 | 96.4 |
| ExtB | 17.4 | 8.0 | 0.4 | 32.2 | 0.4 | 0.4 | 38.5 |
2.3. Interaction of the Derivatized Dendrimers with Red Blood Cells (RBCs)


2.4. In Vivo Effects of the Selected Dendrimers
| Saline | U(VI) | G4-Lys-Fmoc-Cbz | G5-Cou | EDTA | G4-Lys-Fmoc-Cbz + U(VI) | GV5-Cou + U(VI) | EDTA + U(VI) | |
|---|---|---|---|---|---|---|---|---|
| Urea (mg/dL) | 53.3 ± 2.2 | 226.3 ± 39.4 ** | 46.3 ± 4.1 | 43.5 ± 2.2 | 90.0 ± 17.7 | 166.3 ± 20.9 ** | 202.3 ± 31.0 ** | 192.5 ± 4.4 ** |
| Uric Acid (mg/dL) | 0.77 ± 0.15 | 2.5 ± 0.39 ** | 1.4 ± 0.21 | 1.4 ± 0.21 | 2.2 ± 0.4 | 3.3 ± 0.16 ** | 2.8 ± 0.35 ** | 3.2 ± 0.35 ** |
| Calcium (mg/dL) | 7.0 ± 0.23 | 8.8 ± 0.57 * | 7.3 ± 0.42 | 7.3 ± 0.42 | 6.8 ± 0.39 | 8.4 ± 0.31 | 7.9 ± 0.36 | 7.7 ± 0.38 |


3. Experimental Section
3.1. Dendrimer Synthesis
3.1.1. PAMAM G4-Arginine-Tos-OH (G4-Arg-Tos)
3.1.2. PAMAM G4-Lysine-Fmoc-Cbz (G4-Lys-Fmoc-Cbz)
3.1.3. PAMAM G4-Lysine-Cbz-OH (G4-Lys-Cbz)
3.1.4. PAMAM-G5-Folic Acid (G5-FA)
3.1.5. PAMAM-G5-Coumarin (G5-Cou)
3.2. MALDI Analysis
3.3. Affinity of Derivatized Dendrimers to Uranium
3.3.1. Uranium
3.3.2. Affinity Assay
3.4. Red Blood Cells (RBCs) interactions
3.4.1. Sample Collection
3.4.2. Assay of Red Blood Cell Lysis
3.5. In Vivo Acute Toxicity Assay
3.5.1. Animals
3.5.2. Animal Experimental Design
3.6. Biochemistry and Nephrotoxicity
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haley, D.P. Morphologic changes in uranyl nitrate-induced acute renal failure in saline- and water-drinking rats. Lab. Investing. 1982, 46, 196–208. [Google Scholar]
- May, L.M.; Heller, J.; Kalinsky, V.; Ejnik, J.; Cordero, S.; Oberbroekling, K.J.; Long, T.T.; Meakim, K.C.; Cruess, D.; Lee, A.P. Military deployment human exposure assessment: Urine total and isotopic uranium sampling results. J. Toxicol. Environ. Health A 2004, 67, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.S.; Hancock, S.K.; McNally, A.M.; Hinckley, J.; Binder, E.; Zimmerman, K.; Ehrich, M.F.; Jortner, B.S. Neurological effects of acute uranium exposure with and without stress. Neurotoxicology 2007, 28, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.M.; Monette, F.A.; Avci, H.I. Overview of toxicity data and risk assessment methods for evaluating the chemical effects of depleted uranium compounds. Hum. Ecol. Risk Assess. 2000, 6, 851–874. [Google Scholar] [CrossRef]
- Ma, S.; Huang, L.; Ma, L.; Shim, Y.; Islam, S.M.; Wang, P.; Zhao, L.D.; Wang, S.; Sun, G.; Yang, X.; et al. Efficient uranium capture by polysulfide/layered double hydroxide composites. J. Am. Chem. Soc. 2015, 137, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Domingo, J.L.; Gomez, M.; Corbella, J. Treatment of experimental acute uranium poisoning by chelating agents. Pharmacol. Toxicol. 1989, 64, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Ortega, A.; Llobet, J.M.; Corbella, J. Effectiveness of chelation therapy with time after acute uranium intoxication. Toxicol. Sci. 1990, 14, 88–95. [Google Scholar] [CrossRef]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Health Res. 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.Q.; Baker, G.R.; Gupta, V.K. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2006. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Jain, N.K.; McMillan, N.A.; Parekh, H.S. Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine 2010, 6, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bumb, A.; Brechbiel, M.W.; Choyke, P. Macromolecular and dendrimer-based magnetic resonance contrast agents. Acta Radiol. 2010, 51, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Pittelkow, M.; Nielsen, C.B.; Broeren, M.A.; van Dongen, J.L.; van Genderen, M.H.; Meijer, E.W.; Christensen, J.B. Molecular recognition: Comparative study of a tunable host-guest system by using a fluorescent model system and collision-induced dissociation mass spectrometry on dendrimers. Chemistry 2005, 11, 5126–5135. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.L.; Klushin, L.I. Intrinsic viscosity of model starburst dendrimers. J. Phys. Chem. 1992, 96, 3994–3998. [Google Scholar] [CrossRef]
- Flora, S.J.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar] [PubMed]
- Andersen, O. Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 1999, 99, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Duran-Lara, E.F.; Avila-Salas, F.; Galaz, S.; John, A.; Marican, A.; Gutierrez, M.; Nachtigall, F.M.; Gonzalez-Nilo, F.D.; Santos, L.S. Nano-detoxification of organophosphate agents by pamam derivatives. J. Brazil. Chem. Soc. 2015, 26, 580–591. [Google Scholar] [CrossRef]
- Army Environmental Policy Institute (AEPI). Health and Environmental Consequences of Depleted Uranium Use in the Us Army; AEPI: Atlanta, GA, USA, 1995. [Google Scholar]
- The Royal Society. The Health Hazards of Depleted Uranium Munitions Part I; Policy Document 6/01; Royal Society: London, UK, 2001. [Google Scholar]
- The Royal Society. The Health Effects of Depleted Uranium Munitions; Summary; Royal Society: London, UK, 2002. [Google Scholar]
- Leggett, R.W. The behavior and chemical toxicity of u in the kidney: A reassessment. Health Phys. 1989, 57, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.L.; Barber, D.S.; Ehrich, M.F.; Tobias, L.; Hancock, S.; Hinckley, J.; Binder, E.M.; Jortner, B.S. Temporal clinical chemistry and microscopic renal effects following acute uranyl acetate exposure. Toxicol. Pathol. 2007, 35, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.L.; Zielinski, J.M.; Moodie, G.B.; Falcomer, R.A.; Hunt, W.C.; Capello, K. Uranium in drinking water: Renal effects of long-term ingestion by an aboriginal community. Arch. Environ. Occup. Health 2009, 64, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.P.; Moss, M.A.; Villeneuve, D.C.; Secours, V.E.; Yagminas, A.P.; Tracy, B.L.; Quinn, J.M.; Long, G.; Valli, V.E. Uranyl nitrate: 91-day exposure and recovery studies in the male new zealand white rabbit. Toxicol. Sci. 1998, 41, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kumar, A.; Pandey, B.N.; Mishra, K.P. Acute exposure of uranyl nitrate causes lipid peroxidation and histopathological damage in brain and bone of wistar rat. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Durán-Lara, E.; Guzmán, L.; John, A.; Fuentes, E.; Alarcón, M.; Iván, P.; Santos, L.S. Pamam dendrimer derivatives as a potential drug for antithrombotic therapy. Eur. J. Med. Chem. 2013, 69, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, D.A.; Duran-Lara, E.F.; Aguayo, D.; Cachau, R.E.; Tapia, J.; Esparza, R.; Yacaman, M.J.; Gonzalez-Nilo, F.D.; Santos, L.S. Supramolecular complexes of quantum dots and a polyamidoamine (pamam)-folate derivative for molecular imaging of cancer cells. Anal. Bioanal. Chem. 2011, 400, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.S.; Arasho, W.; Johnson, J.H., Jr.; Goddard, W.A., III. Dendritic chelating agents. 2. U(VI) binding to poly(amidoamine) and poly(propyleneimine) dendrimers in aqueous solutions. Environ. Sci. Technol. 2008, 42, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Liu, Y.; Wang, C.; Cheng, J. Phosphorus-modified poly(styrene-co-divinylbenzene)-pamam chelating resin for the adsorption of uranium(vi) in aqueous. J. Hazard. Mater. 2013, 263 (Pt 2), 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ilaiyaraja, P.; Deb, A.K.; Sivasubramanian, K.; Ponraju, D.; Venkatraman, B. Adsorption of uranium from aqueous solution by pamam dendron functionalized styrene divinylbenzene. J. Hazard. Mater. 2013, 250–251, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Matuszko, G.; Bryszewska, M.; Klajnert, B. Influence of dendrimers on red blood cells. Cell Mol. Biol. Lett. 2011, 17, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Blantz, R.C. The mechanism of acute renal failure after uranyl nitrate. J. Clin. Investig. 1975, 55, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Llobet, J.M.; Tomas, J.M.; Corbella, J. Acute toxicity of uranium in rats and mice. Bull. Environ. Contam. Toxicol. 1987, 39, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pisal, D.S.; Yellepeddi, V.K.; Kumar, A.; Kaushik, R.S.; Hildreth, M.B.; Guan, X.; Palakurthi, S. Permeability of surface-modified polyamidoamine (pamam) dendrimers across caco-2 cell monolayers. Int. J. Pharm. 2008, 350, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ku, Y.K.; Brown, G.M. Sorption and desorption of perchlorate and U(VI) by strong-base anion-exchange resins. Environ. Sci. Technol. 2005, 39, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fernandez, J.C.; Chapman, D. Progress in Membrane Biotechnology (Advances in Life Sciences); Packer, L., Ed.; Birkhauser Verlag: Basel, Switzerland, 1991; pp. 253–265. [Google Scholar]
- Sample Availability: Samples of the dendrimer compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, L.; Durán-Lara, E.F.; Donoso, W.; Nachtigall, F.M.; Santos, L.S. In Vivo Nanodetoxication for Acute Uranium Exposure. Molecules 2015, 20, 11017-11033. https://doi.org/10.3390/molecules200611017
Guzmán L, Durán-Lara EF, Donoso W, Nachtigall FM, Santos LS. In Vivo Nanodetoxication for Acute Uranium Exposure. Molecules. 2015; 20(6):11017-11033. https://doi.org/10.3390/molecules200611017
Chicago/Turabian StyleGuzmán, Luis, Esteban F. Durán-Lara, Wendy Donoso, Fabiane M. Nachtigall, and Leonardo S. Santos. 2015. "In Vivo Nanodetoxication for Acute Uranium Exposure" Molecules 20, no. 6: 11017-11033. https://doi.org/10.3390/molecules200611017