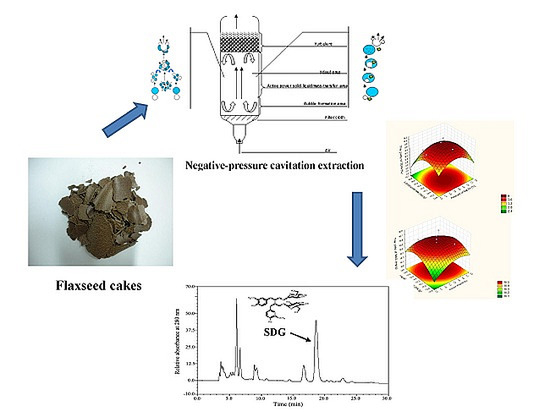
Negative-Pressure Cavitation Extraction of Secoisolariciresinol Diglycoside from Flaxseed Cakes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Significant Parameters Screened by Fractional Factorial Design (FFD)
| Run | Factors | Results | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 a (Mpa) | X2 b (°C) | X3 c (%) | X4 d (%) | X5 e (mL/g) | X6 f (min) | X7 g (L/h) | X8 h (h) | X9 | SDG Yield (mg/g DW) | SDG Purity (%) | |||||||||||||||
| 6 | −0.06(+1) | 20(−1) | 90(+1) | 0.3(−1) | 6(−1) | 60(+1) | 20(−1) | 4(+1) | +1 | 0.07 | 0.05 | ||||||||||||||
| 7 | −0.02(−1) | 50(+1) | 90(+1) | 0.3(−1) | 6(−1) | 10(−1) | 160(+1) | 4(+1) | +1 | 2.04 | 1.06 | ||||||||||||||
| 12 | −0.06(+1) | 50(+1) | 40(−1) | 2.0(+1) | 6(−1) | 10(−1) | 20(−1) | 4(+1) | −1 | 13.56 | 2.75 | ||||||||||||||
| 20(C) | −0.04(0) | 35(0) | 65(0) | 1.15(0) | 13(0) | 35(0) | 90(0) | 2(0) | 0 | 15.17 | 3.09 | ||||||||||||||
| 15 | −0.02(−1) | 50(+1) | 90(+1) | 2.0(+1) | 6(−1) | 60(+1) | 20(−1) | 0(−1) | −1 | 7.36 | 2.75 | ||||||||||||||
| 3 | −0.02(−1) | 50(+1) | 40(−1) | 0.3(−1) | 20(+1) | 60(+1) | 20(−1) | 4(+1) | −1 | 11.96 | 2.77 | ||||||||||||||
| 10 | −0.06(+1) | 20(−1) | 40(−1) | 2.0(+1) | 20(+1) | 60(+1) | 20(−1) | 0(−1) | +1 | 14.11 | 2.66 | ||||||||||||||
| 14 | −0.06(+1) | 20(−1) | 90(+1) | 2.0(+1) | 6(−1) | 10(−1) | 160(+1) | 0(−1) | −1 | 3.32 | 2.43 | ||||||||||||||
| 8 | −0.06(+1) | 50(+1) | 90(+1) | 0.3(−1) | 20(+1) | 10(−1) | 20(−1) | 0(−1) | −1 | 5.17 | 2.78 | ||||||||||||||
| 18(C) | −0.04(0) | 35(0) | 65(0) | 1.15(0) | 13(0) | 35(0) | 90(0) | 2(0) | 0 | 15.38 | 3.47 | ||||||||||||||
| 2 | −0.06(+1) | 20(−1) | 40(−1) | 0.3(−1) | 20(+1) | 10(−1) | 160(+1) | 4(+1) | −1 | 6.56 | 1.94 | ||||||||||||||
| 19(C) | −0.04(0) | 35(0) | 65(0) | 1.15(0) | 13(0) | 35(0) | 90(0) | 2(0) | 0 | 15.66 | 3.53 | ||||||||||||||
| 1 | −0.02(−1) | 20(−1) | 40(−1) | 0.3(−1) | 6(−1) | 10(−1) | 20(−1) | 0(−1) | +1 | 0.25 | 0.08 | ||||||||||||||
| 13 | −0.02(−1) | 20(−1) | 90(+1) | 2.0(+1) | 20(+1) | 10(−1) | 20(−1) | 4(+1) | +1 | 4.80 | 3.54 | ||||||||||||||
| 17(C) | −0.04(0) | 35(0) | 65(0) | 1.15(0) | 13(0) | 35(0) | 90(0) | 2(0) | 0 | 15.23 | 3.20 | ||||||||||||||
| 16 | −0.06(+1) | 50(+1) | 90(+1) | 2.0(+1) | 20(+1) | 60(+1) | 160(+1) | 4(+1) | +1 | 14.79 | 4.80 | ||||||||||||||
| 4 | −0.06(+1) | 50(+1) | 40(−1) | 0.3)−1) | 6(−1) | 60(+1) | 160(+1) | 0(−1) | +1 | 3.88 | 1.30 | ||||||||||||||
| 9 | −0.02(−1) | 20(−1) | 40(−1) | 2.0(+1) | 6(−1) | 60(+1) | 160(+1) | 4(+1) | −1 | 11.88 | 1.48 | ||||||||||||||
| 11 | −0.02(−1) | 50(+1) | 40(−1) | 2.0(+1) | 20(+1) | 10(−1) | 160(+1) | 0(−1) | +1 | 14.89 | 2.34 | ||||||||||||||
| 5 | −0.02(−1) | 20(−1) | 90(+1) | 0.3(−1) | 20(+1) | 60(+1) | 160(+1) | 0(−1) | −1 | 3.03 | 1.66 | ||||||||||||||
| Factor | Statistics Analysis | ||||||||||||||||||||||||
| Effect | Coefficient | t(11) | p | ||||||||||||||||||||||
| Yield | Purity | Yield | Purity | Yield | Purity | Yield | Purity | ||||||||||||||||||
| Mean/Intercept | 8.95556 | 2.383200 | 8.95556 | 2.383200 | 8.76743 | 13.06841 | 0.000003 | 0.000000 | |||||||||||||||||
| X1 | −0.65728 | −0.380250 | −0.32864 | −0.190125 | −0.28777 | −0.93250 | 0.778873 | 0.371096 | |||||||||||||||||
| X2 | 3.70416 | 0.839750 | 1.85208 | 0.419875 | 1.62175 | 2.05934 | 0.133143 | 0.063928 | |||||||||||||||||
| X3 | −4.56276 | 0.470000 | −2.28138 | 0.235000 | −1.99766 | 1.15259 | 0.071088 | 0.273511 | |||||||||||||||||
| X4 | 6.46816 | 1.390750 | 3.23408 | 0.695375 | 2.83188 | 3.41057 | 0.016317 | 0.005819 | |||||||||||||||||
| X5 | 4.12033 | 1.324500 | 2.06017 | 0.662250 | 1.80396 | 3.24810 | 0.098662 | 0.007761 | |||||||||||||||||
| X6 | 2.06056 | 0.066000 | 1.03028 | 0.033000 | 0.90215 | 0.16185 | 0.386303 | 0.874356 | |||||||||||||||||
| X7 | 0.38872 | −0.045500 | 0.19436 | −0.022750 | 0.17019 | −0.11158 | 0.867952 | 0.913166 | |||||||||||||||||
| X8 | 1.70805 | 0.299250 | 0.85404 | 0.149625 | 0.74782 | 0.73386 | 0.470256 | 0.478386 | |||||||||||||||||
2.2. Significant Parameters Optimized by CCD
| Run | Factors | Yield (mg/g DW) | Purity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Block | X4 (%) | X5 (mL/g) | Observed | Predicted | Observed | Predicted | |||
| 1 | 1 | 1.150(−1) | 13.000(−1) | 16.44 | 16.40 | 3.48 | 3.70 | ||
| 2 | 1 | 1.150(−1) | 13.480(+1) | 16.62 | 16.50 | 3.42 | 3.31 | ||
| 3 | 1 | 1.600(+1) | 13.000(−1) | 16.55 | 16.51 | 3.64 | 3.69 | ||
| 4 | 1 | 1.600(+1) | 13.480(+1) | 16.64 | 16.53 | 3.66 | 3.38 | ||
| 5 | 1 | 1.375(0) | 13.240(0) | 16.41 | 16.59 | 3.96 | 3.97 | ||
| 6 | 1 | 1.375(0) | 13.240(0) | 16.65 | 16.59 | 3.97 | 3.97 | ||
| 7 | 2 | 1.057(−1.414) | 13.240(0) | 16.36 | 16.45 | 3.58 | 3.50 | ||
| 8 | 2 | 1.693(1.414) | 13.240(0) | 16.47 | 16.55 | 3.39 | 3.54 | ||
| 9 | 2 | 1.375(0) | 12.901(−1.414) | 16.40 | 16.43 | 3.98 | 3.77 | ||
| 10 | 2 | 1.375(0) | 13.579(1.414) | 16.38 | 16.51 | 3.00 | 3.27 | ||
| 11 | 2 | 1.375(0) | 13.240(0) | 16.57 | 16.59 | 3.99 | 3.97 | ||
| 12 | 2 | 1.375(0) | 13.240(0) | 16.72 | 16.59 | 3.96 | 3.97 | ||
| Factor | Statistics Analysis | ||||||||
| Effect | Coefficient | t(5) | p | ||||||
| Yield | Purity | Yield | Purity | Yield | Purity | Yield | Purity | ||
| Mean/Intercept | 16.58964 | 3.970503 | 16.58964 | 3.970503 | 230.3988 | 33.38118 | 0.000000 | 0.000000 | |
| (1)X4(L) | 0.06809 | 0.031545 | 0.03405 | 0.015772 | 0.6687 | 0.18753 | 0.518951 | 0.858618 | |
| X4(Q) | −0.09305 | −0.454056 | −0.04653 | −0.227028 | −0.8173 | −2.41433 | 0.434551 | 0.060540 | |
| (2)X5(L) | 0.06097 | −0.354486 | 0.03048 | −0.177243 | 0.5987 | −2.10737 | 0.562198 | 0.088918 | |
| X5(Q) | −0.11901 | −0.449047 | −0.05951 | −0.224523 | −1.0454 | −2.38769 | 0.325443 | 0.062566 | |
| 1L by 2L | −0.04456 | 0.041881 | −0.02228 | 0.020940 | −0.3094 | 0.17605 | 0.762011 | 0.867161 | |

2.3. Model Validation and NPCE Pilot-Scale Application
2.4. Comparison of NPCE with Other Extraction Methods
| Method | Extraction Temperature | Extraction Time | Liquid/Solid Ratio (mL/g) | Pressure (MPa) | The yield of SDG (mg/g DW) | The purity of SDG (%) |
|---|---|---|---|---|---|---|
| HRE | 90 °C | 2 h | 20:1 | Atmospheric pressure | 15.44 ± 0.06 a | 2.41 ± 0.19 a |
| LM | 35 °C | 24 h | 30:1 | Atmospheric pressure | 13.29 ± 0.69 b | 3.02 ± 0.31 a |
| UAE | Room temperature | 45 min | 15:1 | Atmospheric pressure | 14.65 ± 0.53 a | 3.92 ± 0.34 b |
| NPCE | 35 °C | 35 min | 13:1 | −0.04 | 15.61 ± 0.53 a | 3.87 ± 0.36 b |
3. Experimental Section
3.1. Reagents and Materials
3.2. Plant Material and Sample Preparation

3.3. NPCE Device

3.4. Extraction Procedures
3.4.1. Lixiviating Method (LM)
3.4.2. Heat Reflux Extraction (HRE)
3.4.3. Ultrasonic Assisted Extraction (UAE)
3.4.4. Negative-Pressure Cavitation Extraction (NPCE)
3.5. The Experimental Design
3.5.1. Fractional Factorial Design (FFD)
3.5.2. Central Composite Design (CCD)
3.6. Analysis of SDG by HPLC
3.7. NPCE Pilot-Scale Experiment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Przybylski, R. Flax oil and high linolenic oils. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; Volume 2, pp. 281–301. [Google Scholar]
- Vijaimohan, K.; Jainu, M.; Sabitha, K.E.; Subramaniyam, S.; Anandhan, C.; Shyamala Devi, C.S. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006, 79, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Natarajan, K.; Matthees, D.P. Chemopreventive effects of dietary flaxseed oil on colon tumor development. Nutr. Cancer 2005, 51, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Flaxseed: A source of hypocholesterolemic and antiatherogenic agents. Drug News Perspect. 2000, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bakke, J.E.; Klosterman, H.J. A new diglucoside from flaxseed. Proc. Nat. Acad. Sci. USA 1956, 10, 18–21. [Google Scholar]
- Prasad, K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside (SDG) isolated from flaxseed. Circulation 1999, 99, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Prasad, K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flaxseed. Mol. Cell. Biochem. 1997, 168, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Rajesha, J.; Murthy, K.N.C.; Karuna, K.M.; Madhusudhan, B.; Ravishankar, G.A. Antioxidant potentials of flaxseed by in vivo model. J. Agric. Food Chem. 2006, 54, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mantha, S.; Muir, A.; Westcott, N. Protective effect of secoisolariciresinol diglucoside against streptozotocin-induced diabetes and its mechanism. Mol. Cell. Biochem. 2000, 206, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, A.; Habben, S.; Winterhalter, P. Isolation of the lignan secoisolariciresinol diglucoside from flaxseed (Linum usitatissimum L.) by high-speed counter-current chromatography. J. Chromatogr. A 2002, 943, 299–302. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.P.; Xu, S.P.; Wang, J.H.; Liu, X. Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flaxseed extract by high-performance liquid chromatography. J. Chromatogr. A 2008, 1185, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. High-performance liquid chromatographic analysis of seoicolariciresinol diglucoside and hydroxycin-namic acid glucosides in flaxseed by alkaline extraction. J. Chromatogr. A 2003, 1012, 151–159. [Google Scholar] [CrossRef]
- Beejmohun, V.; Fliniaux, O.; Grand, E.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.A.; Mesnard, F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem. Anal. 2007, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kraushofer, T.; Sontag, G. Determination of some phenolic compounds in flaxseed and nettle roots by HPLC with coulometric electrode array detection. Eur. Food Res. Technol. 2002, 215, 529–533. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, L.C.W.; Venema, D.P.; Lasaroms, J.J.P.; Wähälä, K.; Hollman, P.C.H. Optimization of a liquid chromatography–tandem mass spectrometry method for quantification of the plant lignans secoisolariciresinol, matairesinol, lariciresinol and pinoresinol in foods. J. Agric. Food Chem. 2004, 52, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.L.; Vorobieva, E. Valorization of oilseed residues: Extraction of polyphenolsfrom flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Boussetta, N.; Turk, M.; De Taeye, C.; Larondellec, Y.; Lanoiselléd, J.L.; Vorobieva, E. Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignansfrom flaxseed cake. Ind. Crops Prod. 2013, 49, 690–696. [Google Scholar] [CrossRef]
- Dong, L.L.; Fu, Y.J.; Zu, Y.G.; Li, J.; Li, X.J.; Efferth, T. Negative pressure cavitation accelerated processing for extraction of main bioactive flavonoids from Radix Scutellariae. Chem. Eng. Process. 2011, 50, 780–789. [Google Scholar] [CrossRef]
- Zhao, B.S.; Fu, Y.J.; Wang, W.; Zu, Y.G.; Gu, C.B.; Luo, M.; Efferth, T. Enhanced extraction of isoflavonoids from Radix Astragali by incubation pretreatment combined with negative pressure cavitation and its antioxidant activity. Innov. Food Sci. Emerg. 2011, 12, 577–585. [Google Scholar] [CrossRef]
- Yan, M.M.; Chen, C.Y.; Zhao, B.S.; Zu, Y.G.; Fu, Y.J.; Liu, W.; Efferth, T. Enhanced extraction of astragalosides from Radix Astragali by negative pressure cavitation-accelerated enzyme pretreatment. Bioresour. Technol. 2010, 101, 7462–7471. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wei, Z.F.; Fu, Y.J.; Gu, C.B.; Zhao, C.J.; Yao, X.H.; Efferth, T. Negative-pressure cavitation extraction of cajaninstilbene acid and pinostrobin from pigeon pea [Cajanus cajan (L.) Millsp.] leaves and evaluation of antioxidant activity. Food Chem. 2011, 128, 596–605. [Google Scholar] [CrossRef]
- Li, X.J.; Yu, H.M.; Gao, C.; Zu, Y.G.; Wang, W.; Luo, M.; Gu, C.B.; Zhao, C.J.; Fu, Y.J. Application of ionic liquid-based surfactants in the microwave-assisted extraction for the determination of four main phloroglucinols from Dryopteris fragrans. J. Sep. Sci. 2012, 35, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, L.Q.; Yao, X.H.; Mu, F.S.; Zhang, D.Y.; Song, Z.Y.; Qiao, Q.; Fu, Y.J.; Zu, Y.G. Optimization of enzyme-assisted negative pressure cavitation extraction of five main indole alkaloids from Catharanthus roseus leaves and its pilot-scale application. Sep. Purif. Technol. 2014, 125, 66–73. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, F.Y.; Gai, Q.Y.; Wang, W.; Luo, M.; Fu, Y.J.; Ma, W. A pilot-scale homogenization-assisted negative pressure cavitationextraction of Astragalus polysaccharides. Int. J. Biol. Macromol. 2014, 67, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fu, Y.J.; Zu, Y.G.; Kong, Y.; Zhang, L.; Zu, B.S.; Efferth, T. Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Gu, C.B.; Wang, W.; Yao, X.H. Negative pressure cavitation extraction and antioxidant activity of biochanin A and genistein from the leaves of Dalbergia odorifera T. Sep. Purif. Technol. 2011, 83, 91–99. [Google Scholar] [CrossRef]
- Dong, L.L.; Fu, Y.J.; Zu, Y.G.; Luo, M.; Wang, W.; Li, X.J.; Li, J. Application of cavitation system to accelerate the endogenous enzymatic hydrolysis of baicalin and wogonoside in Radix Scutellariae. Food Chem. 2012, 131, 1422–1429. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Peerlkamp, N.; Johnsson, P.; Andersson, R.; Andersson, R.E.; Lundgren, L.N.; Åman, P. An oligomer from flaxseed composed of secoisolariciresinol diglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry 2001, 58, 587–590. [Google Scholar] [CrossRef]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Åman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.L.; Cacacea, J.E.; Mazzaa, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT-Food Sci. Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Zhang, W.B.; Xu, S.Y. Microwave-assisted extraction of secoisolariciresinol diglucoside from flaxseed hull. J. Sci. Food Agric. 2007, 87, 1455–1462. [Google Scholar] [CrossRef]
- Nemes, S.M.; Orsat, V. Microwave-assisted extraction of secoisolariciresinol diglucoside-method development. Food Bioprocess Technol. 2011, 4, 1219–1227. [Google Scholar] [CrossRef]
- Li, S.M.; Fu, Y.J.; Zu, Y.G.; Zu, B.; Wang, Y.; Efferth, T. Determination of paclitaxel and its analogues in the needles of Taxus species by using negative pressure cavitation extraction followed by HPLC-MS-MS. J. Sep. Sci. 2009, 32, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Li, D.; Wang, L.J.; Ozkan, N.; Chen, X.D.; Mao, Z.H.; Yang, H.Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Shi, Y.P.; Ma, C.Y. Determination of the Lignan Secoisolariciresinol Diglucoside from Flaxseed (Linum usitatissimum L.) by HPLC. J. Liquid Chromatogr. Relat. Technol. 2007, 30, 533–544. [Google Scholar] [CrossRef]
- Zhuang, Y.P.; Chen, B.; Chu, J.; Zhang, S.L. Medium optimization for meilingmycin production by Streptomyces nanchangensis using response surface methodology. Process Biochem. 2006, 41, 405–409. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Sample Availability: Samples of flaxseed cakes are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Li, W.-Y.; Xiao, D.; Li, Z.-M.; Wang, J.-W. Negative-Pressure Cavitation Extraction of Secoisolariciresinol Diglycoside from Flaxseed Cakes. Molecules 2015, 20, 11076-11089. https://doi.org/10.3390/molecules200611076
Tian H, Li W-Y, Xiao D, Li Z-M, Wang J-W. Negative-Pressure Cavitation Extraction of Secoisolariciresinol Diglycoside from Flaxseed Cakes. Molecules. 2015; 20(6):11076-11089. https://doi.org/10.3390/molecules200611076
Chicago/Turabian StyleTian, Hao, Wan-Yi Li, Dan Xiao, Zhi-Min Li, and Jian-Wen Wang. 2015. "Negative-Pressure Cavitation Extraction of Secoisolariciresinol Diglycoside from Flaxseed Cakes" Molecules 20, no. 6: 11076-11089. https://doi.org/10.3390/molecules200611076