Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening of PIRE
| Phytochemical Content | Mean ± SD |
|---|---|
| Total Flavonoid | 112.36 ± 0.51 mg of CE/g of dry extract |
| Tannin | 27.92 ± 0.81 mg of CE/g of dry extract |
| Total Phenol | 209.21 ± 1.47 mg of GAE/g of dry extract |
| Alkaloid | 32.24 ± 0.38 mg of AE/g of dry extract |
2.2. PIRE Suppresses NPC Cell Proliferation

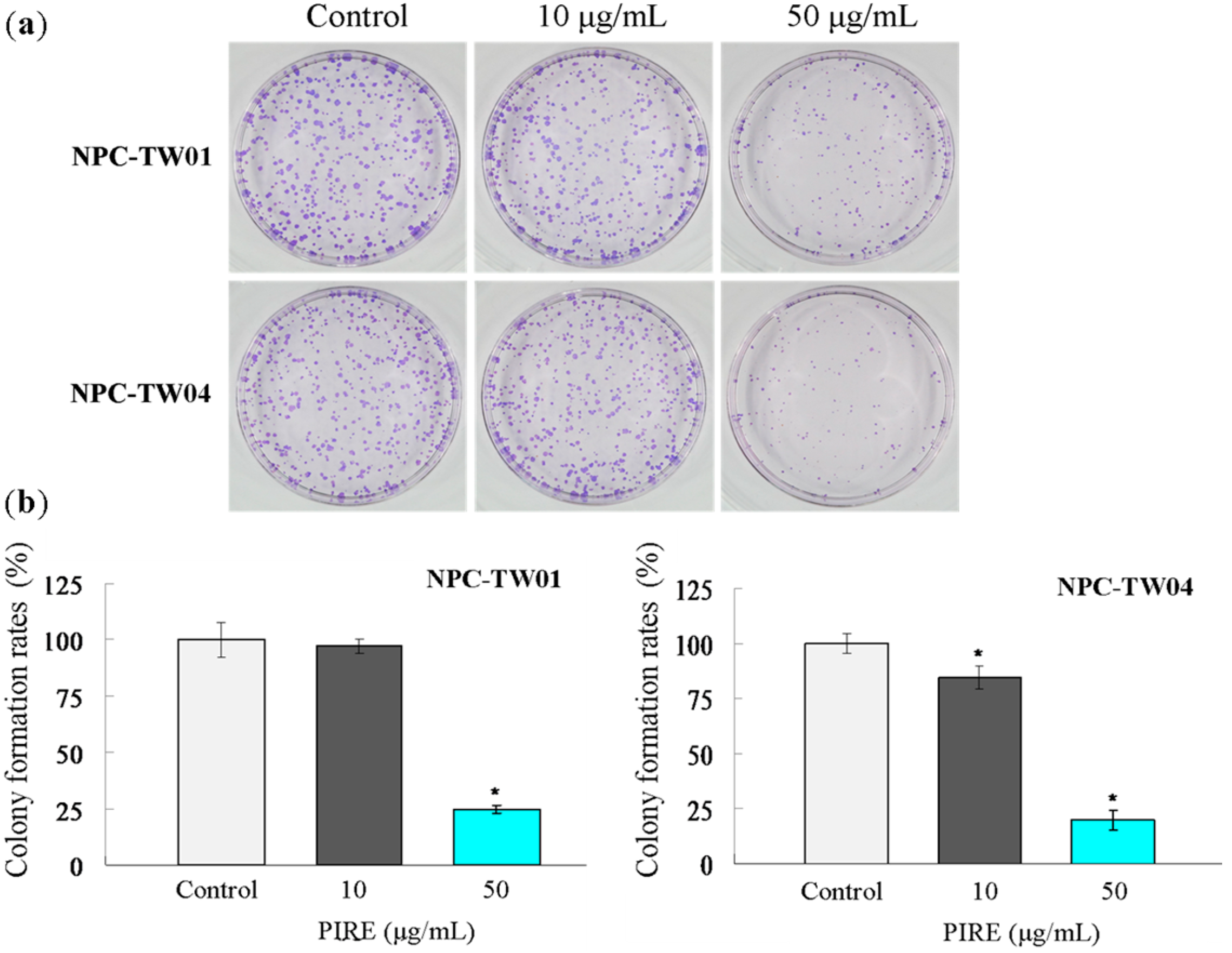
2.3. PIRE Inhibits NPC Cell Migration

2.4. PIRE Induces NPC Cell Apoptosis


2.5. Effect of PIRE on the Expression of Apoptosis-Related Proteins

2.6. Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Preliminary Phytochemical Analysis
3.5. Cell Culture
3.6. Cell Viability Assays
3.7. Colony Formation Assay
3.8. In Vitro Wound Healing Assay
3.9. Migration Assay
3.10. Flow Cytometric Analysis
3.11. TUNEL Staining
3.12. Western Blot Analysis
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, E.T.; Adami, H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Muir, C.S. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci. Publ. 1992, 120, 45–173. [Google Scholar] [PubMed]
- Buell, P. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res. 1974, 34, 1189–1191. [Google Scholar] [PubMed]
- Pegtel, D.M.; Subramanian, A.; Sheen, T.S.; Tsai, C.H.; Golub, T.R.; Thorley-Lawson, D.A. Epstein-Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: Possible role in nasopharyngeal carcinoma metastasis. J. Virol. 2005, 79, 15430–15442. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Nakayama, M.; Nishimura, B.; Hayashi, K.; Hara, A. Early detection of nasopharyngeal carcinoma. Int. J. Otorhinolaryngol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Wei, B.Q.; Chen, W.Z.; Qian, P.D.; Zhang, Y.Q.; Wei, Q.; Cha, W.W.; Li, F.; Ni, M. Staging of nasopharyngeal carcinoma investigated by magnetic resonance imaging. Radiother. Oncol. 2006, 79, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.; Yu, P.; Lee, W.Y.; Leung, S.F.; Kwan, W.H.; Yu, K.H.; Choi, P.; Johnson, P.J. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 291–304. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; LeBlanc, M.; Giri, P.G.; Fu, K.K.; Cooper, J.; Vuong, T.; Forastiere, A.A.; Adams, G.; Sakr, W.A.; Schuller, D.E.; et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998, 16, 1310–1317. [Google Scholar] [PubMed]
- Cheng, S.H.; Tsai, S.Y.; Yen, K.L.; Jian, J.J.; Chu, N.M.; Chan, K.Y.; Tan, T.D.; Cheng, J.C.; Hsieh, C.Y.; Huang, A.T. Concomitant radiotherapy and chemotherapy for early-stage nasopharyngeal carcinoma. J. Clin. Oncol. 2000, 18, 2040–2045. [Google Scholar] [PubMed]
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahman, M.R.; Abdul Razak, F.; Mohd Bakri, M. Evaluation of wound closure activity of Nigella sativa, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum Extracts on scratched monolayer of human gingival fibroblasts. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Saha, A.; Chatterjee, I.; Chakravarty, A.K. Viper and cobra venom neutralization by beta-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae). Phytomedicine 2007, 14, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Nag Chaudhuri, A.K. Antiinflammatory evaluation of a Pluchea indica root extract. J. Ethnopharmacol. 1991, 33, 135–141. [Google Scholar] [CrossRef]
- Buapool, D.; Mongkol, N.; Chantimal, J.; Roytrakul, S.; Srisook, E.; Srisook, K. Molecular mechanism of anti-inflammatory activity of Pluchea indica leaves in macrophages RAW 264.7 and its action in animal models ofinflammation. J. Ethnopharmacol. 2013, 146, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Dhara, A.K.; Bhattacharjee, S.; Pal, S.; Nag Chaudhuri, A.K. Antioxidant activity of the methanol fraction of Pluchea indica root extract. Phytother. Res. 2002, 16, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Ghosh, T.K.; Chaudhuri, A.K. Studies on the mechanism of anti-inflammatory and anti-ulcer activity of Pluchea indica—probable involvement of 5-lipooxygenase pathway. Life Sci. 1993, 52, 737–743. [Google Scholar] [CrossRef]
- Locher, C.P.; Witvrouw, M.; De Bethune, M.P.; Burch, M.T.; Mower, H.F.; Davis, H.; Lasure, A.; Pauwels, R.; de Clercq, E.; Vlietinck, A.J. Antiviral activity of Hawaiian medicinal plants against human immunodeficiency Virus Type-1 (HIV-1). Phytomedicine 1996, 2, 259–264. [Google Scholar] [CrossRef]
- Park, H.-R.; Lee, H.-S.; Cho, S.Y.; Kim, Y.-S.; Shin, K.-S. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int. J. Mol. Med. 2013, 31, 361–368. [Google Scholar] [PubMed]
- Karna, P.; Gundala, S.R.; Gupta, M.V.; Shamsi, S.A.; Pace, R.D.; Yates, C.; Narayan, S.; Aneja, R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis 2011, 32, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Satomi, Y.; Tokuda, H.; Masuda, M. Cancer control by phytochemicals. Curr. Pharm. Des. 2007, 13, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, G.; Venditti, A.; del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Cox, M.C.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003, 101, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q. Restoring p53-mediated apoptosis in cancer cells: New opportunities for cancer therapy. Drug Resist. Updat. 2006, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Zin, N.M.; Wahab, H.A.; Ibrahim, P.; Sulaiman, S.F.; Zahariluddin, A.S.; Noor, S.S. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J. Ethnopharmacol. 2011, 133, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Arsiningtyas, I.S.; Gunawan-Puteri, M.D.; Kato, E.; Kawabata, J. Identification of alpha-glucosidase inhibitors from the leaves of Pluchea indica (L.) Less., a traditional Indonesian herb: Promotion of natural product use. Nat. Prod. Res. 2014, 28, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Oda, Y.; Yamamoto, H.; Uryu, H.; Shiratsuchi, H.; Hirakawa, N.; Tomita, K.; Yamamoto, T.; Oda, S.; Yamada, T.; et al. Overexpression of inducible nitric oxide synthase and accumulation of 8-OHdG in nasopharyngeal carcinoma. Histopathology 2008, 52, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Sato, H.; Murono, S.; Furukawa, M.; Yoshizaki, T. Epstein-Barr virus (EBV) latent membrane protein 1 induces interleukin-8 through the nuclear factor-kappa B signaling pathway in EBV-infected nasopharyngeal carcinoma cell line. Laryngoscope 2004, 114, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, M.-M.; Han, P.; Lin, J.-Z.; Liang, F.-Y.; Tan, G.-M.; Li, H.; Zeng, M.; Huang, X. Id-1 and the p65 subunit of NF-kappaB promote migration of nasopharyngeal carcinoma cells and are correlated with poor prognosis. Carcinogenesis 2012, 33, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.J.; Cho, C.L.; Kao, C.L.; Chen, C.M.; Tseng, C.N.; Lee, Y.Z.; Liao, L.J.; Hong, Y.R. Crude aqueous extracts of Pluchea indica (L.) Less. inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Stefani, S. Distant metastases of nasopharyngeal carcinoma: A study of 256 male patients. J. Surg. Oncol. 1986, 33, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Peng, L.-X.; Shao, J.-Y.; Lu, W.-H.; Zhang, J.-X.; Chen, S.; Chen, Z.-Y.; Xiang, Y.-Q.; Bao, Y.-N.; Zheng, F.-J.; et al. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis 2012, 33, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Geara, F.B.; Sanguineti, G.; Tucker, S.L.; Garden, A.S.; Ang, K.K.; Morrison, W.H.; Peters, L.J. Carcinoma of the nasopharynx treated by radiotherapy alone: Determinants of distant metastasis and survival. Radiother. Oncol. 1997, 43, 53–61. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Ye, Q.; Yang, Z.; Dong, Z. Study of the relation between MMP2, MMP9 and nasopharyngeal carcinoma. J. Clin. Otolaryngol. 1999, 13, 356–358. [Google Scholar]
- Sun, B.; Xu, M. Matrine inhibits the migratory and invasive properties of nasopharyngeal carcinoma cells. Mol. Med. Rep. 2015, 11, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Yokosawa, E.; Koyano, T.; Preeprame, S.; Kowithayakorn, T.; Sakai, S.; Toida, T.; Ishibashi, M. Quinic acid esters from Pluchea indica with collagenase, MMP-2 and MMP-9 inhibitory activities. Phytother. Res. 2008, 22, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
- Hock, A.K.; Vousden, K.H. Tumor suppression by p53: Fall of the triumvirate? Cell 2012, 149, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.; Cooper, G.M. p53 is a major component of the transcriptional and apoptotic program regulated by PI 3-kinase/Akt/GSK3 signaling. Cell Death Differ. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, J.; Schlosser, S.; Muller, M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Beckerman, R.; Prives, C. Transcriptional regulation by p53. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef]
- Elkholi, R.; Floros, K.V.; Chipuk, J.E. The role of BH3-only proteins in tumor cell development, signaling, and treatment. Genes Cancer 2011, 2, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.-Q.; Yi, H.; Li, X.-H.; Shi, H.-Y.; Li, C.; Li, M.-Y.; Zhang, P.-F.; Feng, X.-P.; Wan, X.-X.; Qu, J.-Q.; et al. Identification of the proteins related to p53-mediated radioresponse in nasopharyngeal carcinoma by proteomic analysis. J. Proteomics 2011, 74, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, S.; Chen, C.; Xiao, S.; Sun, Y.; Liu, C.; Su, X.; Li, D.; Xu, G.; Xu, B.; et al. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009, 27, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Bustos, R.; Alonso-Castro, A.J.; Gomez-Sanchez, M.; Salazar-Olivo, L.A. Ibervillea sonorae (Cucurbitaceae) induces the glucose uptake in human adipocytes by activating a PI3K-independent pathway. J. Ethnopharmacol. 2014, 152, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Lu, T.Y.; Huang, D.Y.; Kuo, Y.S.; Kao, C.F.; Yeh, N.H.; Wu, H.C.; Lin, C.T. NOLC1, an enhancer of nasopharyngeal carcinoma progression, is essential for TP53 to regulate MDM2 expression. Am. J. Pathol. 2009, 175, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, H.; Shimizu, K. Involvement of Akt/NF-kappaB pathway in antitumor effects of parthenolide on glioblastoma cells in vitro and in vivo. BMC Cancer 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the PIRE extracts are available from the corresponding authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-L.; Cho, J.; Lee, Y.-Z.; Cheng, Y.-B.; Chien, C.-Y.; Hwang, C.-F.; Hong, Y.-R.; Tseng, C.-N.; Cho, C.-L. Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells. Molecules 2015, 20, 11508-11523. https://doi.org/10.3390/molecules200611508
Kao C-L, Cho J, Lee Y-Z, Cheng Y-B, Chien C-Y, Hwang C-F, Hong Y-R, Tseng C-N, Cho C-L. Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells. Molecules. 2015; 20(6):11508-11523. https://doi.org/10.3390/molecules200611508
Chicago/Turabian StyleKao, Chiu-Li, Joshua Cho, Ya-Zhe Lee, Yuan-Bin Cheng, Chih-Yen Chien, Chung-Feng Hwang, Yi-Ren Hong, Chao-Neng Tseng, and Chung-Lung Cho. 2015. "Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells" Molecules 20, no. 6: 11508-11523. https://doi.org/10.3390/molecules200611508
APA StyleKao, C.-L., Cho, J., Lee, Y.-Z., Cheng, Y.-B., Chien, C.-Y., Hwang, C.-F., Hong, Y.-R., Tseng, C.-N., & Cho, C.-L. (2015). Ethanolic Extracts of Pluchea indica Induce Apoptosis and Antiproliferation Effects in Human Nasopharyngeal Carcinoma Cells. Molecules, 20(6), 11508-11523. https://doi.org/10.3390/molecules200611508








