Protective Effects of Dihydrocaffeic Acid, a Coffee Component Metabolite, on a Focal Cerebral Ischemia Rat Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Effect of DHCA on Brain Infarct Volume and Sensory-Motor Function Deficit

2.1.2. Effect of DHCA on BWC and EB Extravasation

2.1.3. Effect of DHCA on MMP-2 and MMP-9 Protein Expression
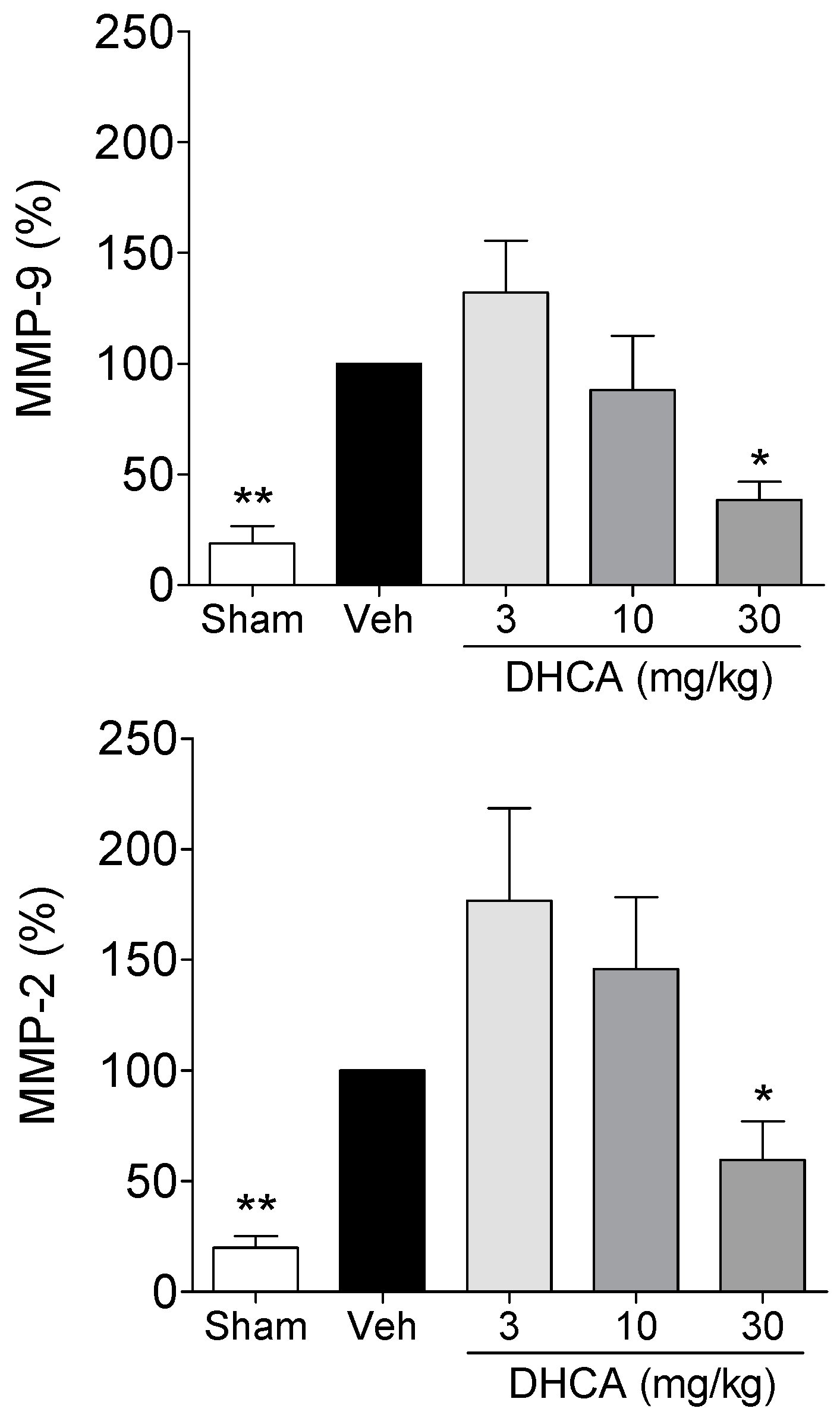
2.1.4. Effect of DHCA on MMP-2 and MMP-9 Activity

2.2. Discussion
3. Experimental Section
3.1. DHCA Preparation
3.2. Animals
3.3. Focal Cerebral Ischemia Rat Model Induction and Treatment
3.4. Balance Beam Test
3.5. Measurement of Infarct Volume
3.6. Measurement of BWC
3.7. Measurement of EB Extravasation
3.8. Western Blot
3.9. In Vitro Zymography
3.10. Statistical Analysis
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [PubMed]
- Bonita, J.S.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007, 55, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.S.; Jang, H.J.; Kim, S.M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Moridani, M.Y.; Scobie, H.; Jamshidzadeh, A.; Salehi, P.; O’Brien, P.J. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: Glutathione conjugate formation. Drug Metab. Dispos. 2001, 29, 1432–1439. [Google Scholar] [PubMed]
- Ludwig, I.A.; Paz de Pena, M.; Concepcion, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Redeuil, K.; Smarrito-Menozzi, C.; Guy, P.; Rezzi, S.; Dionisi, F.; Williamson, G.; Nagy, K.; Renouf, M. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 4678–4688. [Google Scholar] [CrossRef] [PubMed]
- Altug, M.E.; Serarslan, Y.; Bal, R.; Kontas, T.; Ekici, F.; Melek, I.M.; Aslan, H.; Duman, T. Caffeic acid phenethyl ester protects rabbit brains against permanent focal ischemia by antioxidant action: A biochemical and planimetric study. Brain Res. 2008, 1201, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Elango, C.; Ansari, M.A.; Singh, I.; Singh, A.K. Caffeic acid phenethyl ester reduces neurovascular inflammation and protects rat brain following transient focal cerebral ischemia. J. Neurochem. 2007, 102, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Hunneche, C.S.; Lund, M.N.; Skibsted, L.H.; Nielsen, J. Antioxidant activity of a combinatorial library of emulsifier-antioxidant bioconjugates. J. Agric. Food Chem. 2008, 56, 9258–9268. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human ea.Hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Liu, X.; Gong, H.; Huang, L.; Chen, H.; Zhang, X.; Li, C.; Yang, M.; Ma, B.; Jiao, L.; et al. Coffee components inhibit amyloid formation of human islet amyloid polypeptide in vitro: Possible link between coffee consumption and diabetes mellitus. J. Agric. Food Chem. 2011, 59, 13147–13155. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, K.; Omichi, J.; Ohnishi, R.; Ito, H.; Yoshida, T.; Kamei, C. Effects of chlorogenic acid and its metabolites on the sleep-wakefulness cycle in rats. Eur. J. Pharmacol. 2004, 504, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Krafft, P.R.; Bailey, E.L.; Lekic, T.; Rolland, W.B.; Altay, O.; Tang, J.; Wardlaw, J.M.; Zhang, J.H.; Sudlow, C.L. Etiology of stroke and choice of models. Int. J. Stroke 2012, 7, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Zhang, S.M.; Yan, W.M.; Li, X.R.; Zhang, H.Y.; Zheng, X.X. Development of cerebral infarction, apoptotic cell death and expression of x-chromosome-linked inhibitor of apoptosis protein following focal cerebral ischemia in rats. Life Sci. 2006, 78, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [PubMed]
- Gotoh, O.; Asano, T.; Koide, T.; Takakura, K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125i-albumin. Stroke 1985, 16, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Kent, T.A.; Chen, M.; Tarasov, K.V.; Gerzanich, V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007, 6, 258–268. [Google Scholar] [CrossRef]
- Gerriets, T.; Walberer, M.; Ritschel, N.; Tschernatsch, M.; Mueller, C.; Bachmann, G.; Schoenburg, M.; Kaps, M.; Nedelmann, M. Edema formation in the hyperacute phase of ischemic stroke. Laboratory investigation. J. Neurosurg. 2009, 111, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, J.W.; Lee, B.; Bu, Y. Pathologic factors of brain edema in acute ischemic stroke research. Orient. Pharm. Exp. Med. 2015, 15, 1–5. [Google Scholar] [CrossRef]
- Bu, Y.; Lee, K.; Jung, H.S.; Moon, S.K. Therapeutic effects of traditional herbal medicine on cerebral ischemia: A perspective of vascular protection. Chin. J. Integr. Med. 2013, 19, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.; Li, G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 2010, 38, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.A.; Wetzel, M.; Rosenberg, G.A. Multiple roles for mmps and timps in cerebral ischemia. Glia 2005, 50, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 29, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix metalloproteinases and timps are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 1998, 29, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Morancho, A.; Rosell, A.; Garcia-Bonilla, L.; Montaner, J. Metalloproteinase and stroke infarct size: Role for anti-inflammatory treatment? Ann. N. Y. Acad. Sci. 2010, 1207, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lehner, C.; Gehwolf, R.; Tempfer, H.; Krizbai, I.; Hennig, B.; Bauer, H.C.; Bauer, H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal. 2011, 15, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Gasche, Y.; Copin, J.C.; Sugawara, T.; Fujimura, M.; Chan, P.H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.K.; Kim, C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of euonymus alatus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Kim, S.H.; Kim, C.H. A new matrix metalloproteinase-9 inhibitor 3,4-dihydroxycinnamic acid (caffeic acid) from methanol extract of euonymus alatus: Isolation and structure determination. Toxicology 2005, 207, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ngameni, B.; Touaibia, M.; Patnam, R.; Belkaid, A.; Sonna, P.; Ngadjui, B.T.; Annabi, B.; Roy, R. Inhibition of mmp-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of dorstenia turbinata. Phytochemistry 2006, 67, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Kwon, S.; Kim, Y.T.; Kim, M.Y.; Choi, H.; Kim, J.G.; Jamarkattel-Pandit, N.; Dore, S.; Kim, S.H.; Kim, H. Neuroprotective effect of ht008–1, a prescription of traditional korean medicine, on transient focal cerebral ischemia model in rats. Phytother. Res. 2010, 24, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal. Bbiochem. 1994, 218, 325–329. [Google Scholar] [CrossRef]
- Sample Availability: Samples of dihydrocaffeic acid are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Lee, B.-J.; Bu, Y. Protective Effects of Dihydrocaffeic Acid, a Coffee Component Metabolite, on a Focal Cerebral Ischemia Rat Model. Molecules 2015, 20, 11930-11940. https://doi.org/10.3390/molecules200711930
Lee K, Lee B-J, Bu Y. Protective Effects of Dihydrocaffeic Acid, a Coffee Component Metabolite, on a Focal Cerebral Ischemia Rat Model. Molecules. 2015; 20(7):11930-11940. https://doi.org/10.3390/molecules200711930
Chicago/Turabian StyleLee, Kyungjin, Beom-Joon Lee, and Youngmin Bu. 2015. "Protective Effects of Dihydrocaffeic Acid, a Coffee Component Metabolite, on a Focal Cerebral Ischemia Rat Model" Molecules 20, no. 7: 11930-11940. https://doi.org/10.3390/molecules200711930




