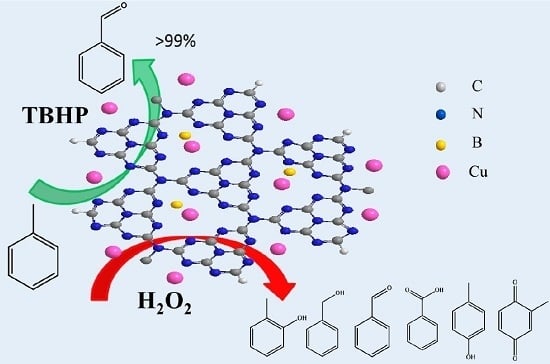
Cu and Boron Doped Carbon Nitride for Highly Selective Oxidation of Toluene to Benzaldehyde
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance of the Cu-CNB Catalyst for the Selective Oxidation of Toluene to Benzaldehyde
| Entry | Catalysts | ntoluene/nTBHP | Temperature/°C | C/% | S/% |
|---|---|---|---|---|---|
| 1 | -- | 1:2.5 | 70 | 0 | 0 |
| 2 | C3N4 | 1:2.5 | 70 | 0 | 0 |
| 3 | CNB | 1:2.5 | 70 | 0 | 0 |
| 4 | Cu-C | 1:2.5 | 70 | 1.42 | >99 |
| 5 | Cu-C3N4 | 1:2.5 | 70 | 1.59 | >99 |
| 6 | Cu-CNB | 1:2.5 | 70 | 4.3 | >99 |
| 7 | Cu-CNB | 1:2.5 | 80 | 4.8 | >99 |
| 8 | Cu-CNB | 1:4 | 70 | 5.5 | >99 |
| 9 b | Cu-CNB | 1:4 | 70 | 6.3 | >99 |

| Entry | Catalyst/mg | Time/h | T/°C | Selectivity/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| BOL | BAL | BAC | o-cresol | p-cresol | MPB | ||||
| 1 | 20 | 20 | 70 | 8.5 | 37 | 31.2 | 10.9 | 7.1 | 5.3 |
| 2 | 30 | 20 | 70 | 17.7 | 61.7 | 0 | 10.5 | 6.3 | 3.9 |
| 3 | 50 | 20 | 70 | 10.8 | 38.7 | 22.8 | 11.9 | 8.1 | 7.7 |
| 4 | 90 | 20 | 70 | 6.7 | 36.1 | 19.5 | 15.6 | 13.3 | 8.9 |
| 5 | 30 | 8 | 70 | 18 | 52.1 | 5.7 | 9.3 | 7.3 | 7.6 |
| 6 | 30 | 16 | 70 | 17.1 | 61.5 | 0 | 8.1 | 6.3 | 7.1 |
| 7 | 30 | 24 | 70 | 17.3 | 63.7 | 0 | 8.5 | 6.8 | 3.7 |
| 8 b | 30 | 24 | 70 | 23.4 | 37.7 | 28 | 2.8 | 0 | 8.1 |
| 9 | 30 | 4 | 40 | 23.1 | 18.8 | 0 | 34 | 24.1 | 0 |
| 10 | 30 | 4 | 60 | 7.6 | 36.4 | 0 | 26.6 | 20.7 | 8.7 |
| 11 | 30 | 4 | 70 | 14.9 | 46.3 | 14.2 | 9.4 | 7.6 | 7.7 |

2.2. Structure Characterization of the Cu-CNB Catalyst



3. Experimental Section
3.1. General Information
3.2. Preparation of the Catalyst Cu-CNB
3.3. Procedures for the Selective Oxidation of Toluene to Benzaldehyde
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Labinger, J.A.; Bercaw, J.E. Understanding and Exploiting C-H Bond Activation. Nature 2002, 417, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Mas-Ballesté, R.; Que, L., Jr. Targeting Specific C-H Bonds for Oxidation. Science 2006, 312, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.G.; Kitamura, T.; Fujiwara, Y. Catalytic Functionalization of Arenes and Alkanes via C-H Bond Activation. Acc. Chem. Res. 2001, 34, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nam, W. Dioxygen Activation by Metalloenzymes and Models. In Accounts Chemical Research; American Chemical Society: Washington, DC, USA, 2007; Volume 40, pp. 465–634. [Google Scholar]
- Zhang, J.; Liu, X.; Blume, R.; Zhang, A.H.; Schlögl, R.; Su, D.S. Surface-Modified Carbon Nanotubes Catalyze Oxidative Dehydrogenation of N-Butane. Science 2008, 322, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; White, M.C. Combined Effects on Selectivity in Fe-Catalyzed Methylene Oxidation. Science 2010, 327, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Partenheimer, W. Methodology and Scope of Metal/Bromide Autoxidation of Hydrocarbons. Catal. Today 1995, 23, 69–158. [Google Scholar] [CrossRef]
- Kesavan, L.; Tiruvalam, R.; Ab Rahim, M.H.; bin Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-Free Oxidation of Primary Carbon-hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles. Science 2011, 331, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Bin Saiman, M.I.; Brett, G.L.; Tiruvalam, R.; Forde, M.M.; Sharples, K.; Thetford, A.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Murphy, D.M.; et al. Involvement of Surface-Bound Radicals in the Oxidation of Toluene Using Supported Au-Pd Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lv, J.; Shen, Y.; Guo, X.; Peng, L.; Xie, Z.; Ding, W. Hexadecylphosphate-Functionalized Iron Oxide Nanoparticles: Mild Oxidation of Benzyl C-H Bonds Exclusive to Carbonyls by Molecular Oxygen. ACS Catal. 2014, 4, 2746–2752. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Tiwari, R.; Sarkar, B.; Singha, R.K.; Pendem, C.; Sasaki, T.; Bal, R. Preparation of the CuCr2O4 Spinel Nanoparticles Catalyst for Selective Oxidation of Toluene to Benzaldehyde. Green Chem. 2014, 16, 2500–2508. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Li, X.; Gao, J.; Zhou, L.; Ohnishi, R. Liquid Phase Oxidation of Toluene to Benzaldehyde with Molecular Oxygen over Copper-Based Heterogeneous Catalysts. Adv. Synth. Catal. 2005, 347, 1987–1992. [Google Scholar] [CrossRef]
- Konietzni, F.; Kolb, U.; Dingerdissen, U.; Maier, W.F. AMM-MnxSi-Catalyzed Selective Oxidation of Toluene. J. Catal. 1998, 176, 527–535. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Chen, J.S.; Wang, X.; Sun, J.; Antonietti, M. Metal-Free Activation of Dioxygen by Graphene/g-C3N4 Nanocomposites: Functional Dyads for Selective Oxidation of Saturated Hydrocarbons. J. Am. Chem. Soc. 2011, 133, 8074–8077. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: from Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wang, X.; Antonietti, M.; Li, H. Boron- and Fluorine-containing Mesoporous Carbon Nitride Polymers: Metal-Free Catalysts for Cyclohexane Oxidation. Angew. Chem. Int. Ed. 2010, 49, 3356–3359. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Villa, A.; Veith, G.M.; Kailasam, K.; Adamczyk, L.A.; Unocic, R.R.; Prati, L.; Thomas, A. Influence of Periodic Nitrogen Functionality on the Selective Oxidation of Alcohols. Chem. Asian J. 2012, 7, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhou, Y.; Chen, G.; Ge, W.; Wang, J. C3N4-H5PMo10V2O40: A Dual-catalysis System for Reductant-Free Aerobic Oxidation of Benzene to Phenol. Sci. Rep. 2014, 4, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Carabineiro, S.A.C.; Shan, D.; Faria, J.L.; Zhu, Y.; Figueiredo, J.L. Oxygen Activation Sites in Gold and Iron Catalysts Supported on Carbon Nitride and Activated Carbon. J. Catal. 2010, 274, 207–214. [Google Scholar] [CrossRef]
- Zhang, P.F.; Gong, Y.T.; Li, H.R.; Chen, Z.R.; Wang, Y. Solvent-free Aerobic Oxidation of Hydrocarbons and Alcohols with Pd@N-doped Carbon from Glucose. Nat. Commun. 2013, 4, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Yao, J.; Wang, X.; Antonietti, M. Synthesis of Boron Doped Polymeric Carbon Nitride Solids and Their Use as Metal-Free Catalysts for Aliphatic C-H Bond Oxidation. Chem. Sci. 2011, 2, 446–450. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, X.; Antonietti, M. Solvent-Free and Metal-Free Oxidation of Toluene Using O2 and g-C3N4 with Nanopores: Nanostructure Boosts the Catalytic Selectivity. ACS Catal. 2012, 2, 2082–2086. [Google Scholar] [CrossRef]
- Ding, G.D.; Wang, W.T.; Jiang, T.; Han, B.X.; Fan, H.L.; Yang, G.Y. Highly Selective Synthesis of Phenol from Benzene over a Vanadium-Doped Graphitic Carbon Nitride Catalyst. ChemCatChem 2013, 5, 192–200. [Google Scholar] [CrossRef]
- Chanquía, C.M.; Cánepa, A.L.; Bazán-Aguirre, J.; Sapag, K.; Rodríguez-Castellón, E.; Reyes, P.; Herrero, E.R.; Casuscelli, S.G.; Eimer, G.A. Copper-Containing Spherical Mesoporous Silicates Prepared by Template-Ion Exchange: A Multitechnique Characterization and Oxidation Properties. Microporus Mesoporus Mater. 2012, 151, 2–12. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Chanquía, C.M.; Eimer, G.A.; Casuscelli, S.G. Oxidation of Olefins Employing Mesoporous Molecular Sieves Modified with Copper. Appl. Catal. A 2013, 462–463, 8–14. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, L.; Jiang, J.; Tang, J.; Cao, X.; Gu, H. CuO@Ag as a Highly Active Catalyst for the Selective Oxidation of Trans-stilbene and Alcohols. Catal. Sci. Technol. 2012, 2, 1146–1149. [Google Scholar] [CrossRef]
- Zhu, M.; Diao, G. High Catalytic Activity of CuO Nanorods for Oxidation of Cyclohexene to 2-cyclohexene-1-one. Catal. Sci. Technol. 2012, 2, 82–84. [Google Scholar] [CrossRef]
- Kanzaki, H.; Kitamura, T.; Hamada, R.; Nishiyama, S.; Tsuruya, S. Activities for Phenol Formation Using Cu Catalysts Supported on Al2O3 in the Liquid-phase Oxidation of Benzene in Aqueous Solvent with High Acetic Acid Concentration. J. Mol. Catal. A -Chem. 2004, 208, 203–211. [Google Scholar] [CrossRef]
- Kiwi-Minsker, L.; Bulushev, D.A.; Rainone, F.; Renken, A. Implication of the Acid-base Properties of V/Ti-oxide Catalyst in Toluene Partial Oxidation. J. Mol. Catal. A 2002, 184, 223–235. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, H.; Wang, Y. Graphitic Carbon Nitride Polymers: Promising Catalysts or Catalyst Supports for Heterogeneous Oxidation and Hydrogenation. Green Chem. 2015, 17, 715–736. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient Synthesis of Polymeric g-C3N4 Layered Materials as Novel Efficient Visible Light Driven Photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, X.; Antonietti, M.; Wang, X. Synthesis of Transition Metal-Modified Carbon Nitride Polymers for Selective Hydrocarbon Oxidation. ChemSusChem 2011, 4, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Jiang, Z.Y.; Geng, J.Q.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Lin, Z.Z.; Wang, X.C. Ionic Liquid Promoted Synthesis of Conjugated Carbon Nitride Photocatalysts from Urea. ChemSusChem 2014, 7, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Wentzcovitch, R.M.; Cohen, M.L. Atomic Arrangement and Electronic Structure of BC2N. Phys. Rev. B 1989, 39, 1760–1765. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Ding, G.; Wu, T.; Yang, D.; Jiang, T.; Han, B. Cu and Boron Doped Carbon Nitride for Highly Selective Oxidation of Toluene to Benzaldehyde. Molecules 2015, 20, 12686-12697. https://doi.org/10.3390/molecules200712686
Han H, Ding G, Wu T, Yang D, Jiang T, Han B. Cu and Boron Doped Carbon Nitride for Highly Selective Oxidation of Toluene to Benzaldehyde. Molecules. 2015; 20(7):12686-12697. https://doi.org/10.3390/molecules200712686
Chicago/Turabian StyleHan, Hongling, Guodong Ding, Tianbin Wu, Dexin Yang, Tao Jiang, and Buxing Han. 2015. "Cu and Boron Doped Carbon Nitride for Highly Selective Oxidation of Toluene to Benzaldehyde" Molecules 20, no. 7: 12686-12697. https://doi.org/10.3390/molecules200712686