Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver
Abstract
:1. Introduction

2. Results and Discussion
2.1. Component Analysis of Licorice Extract
| Sample (Parts) | Solvent | GA (%) | GB (%) | LQ (%) | LG (%) |
|---|---|---|---|---|---|
| 1 (root twigs) | Water | 0.75 ± 0.02 | ND 1 | 0.26 ± 0.02 | 0.07 ± 0.01 |
| 2 (root twigs) | 70% EtOH | 1.68 ± 0.09 | 0.16 ± 0.02 | 1.09 ± 0.04 | 0.08 ± 0.01 |
| 3 (root intercept) | Water | 0.51 ± 0.01 | ND 1 | 0.15 ± 0.01 | 0.04 ± 0.001 |
| 4 (root intercept) | 70% EtOH | 1.09 ± 0.01 | 0.08 ± 0.01 | 0.50 ± 0.01 | 0.01 ± 0.002 |
2.2. HPLC-MS Analysis of Licorice Extracts

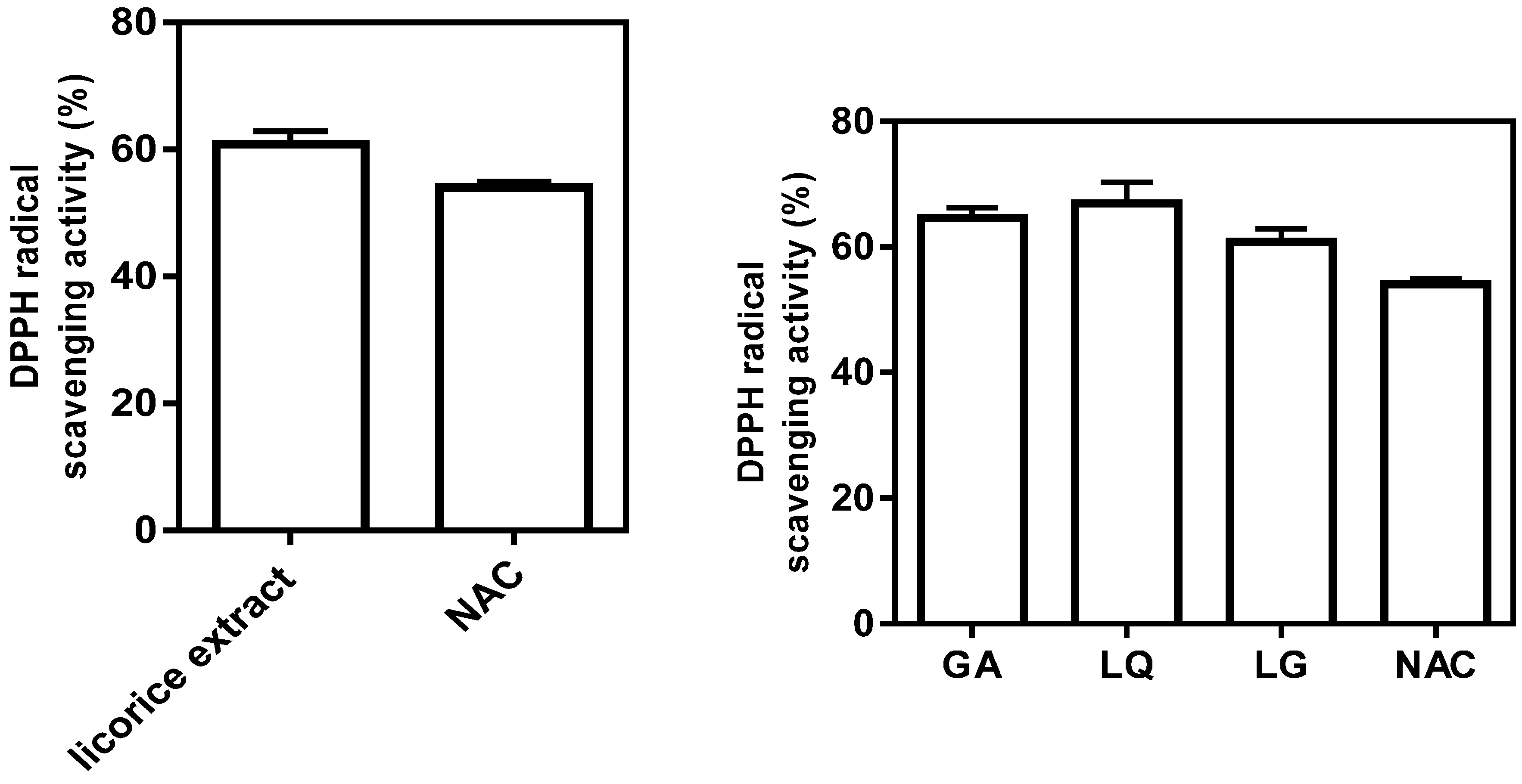
2.3. Antioxidative Activity of Licorice Ingredients
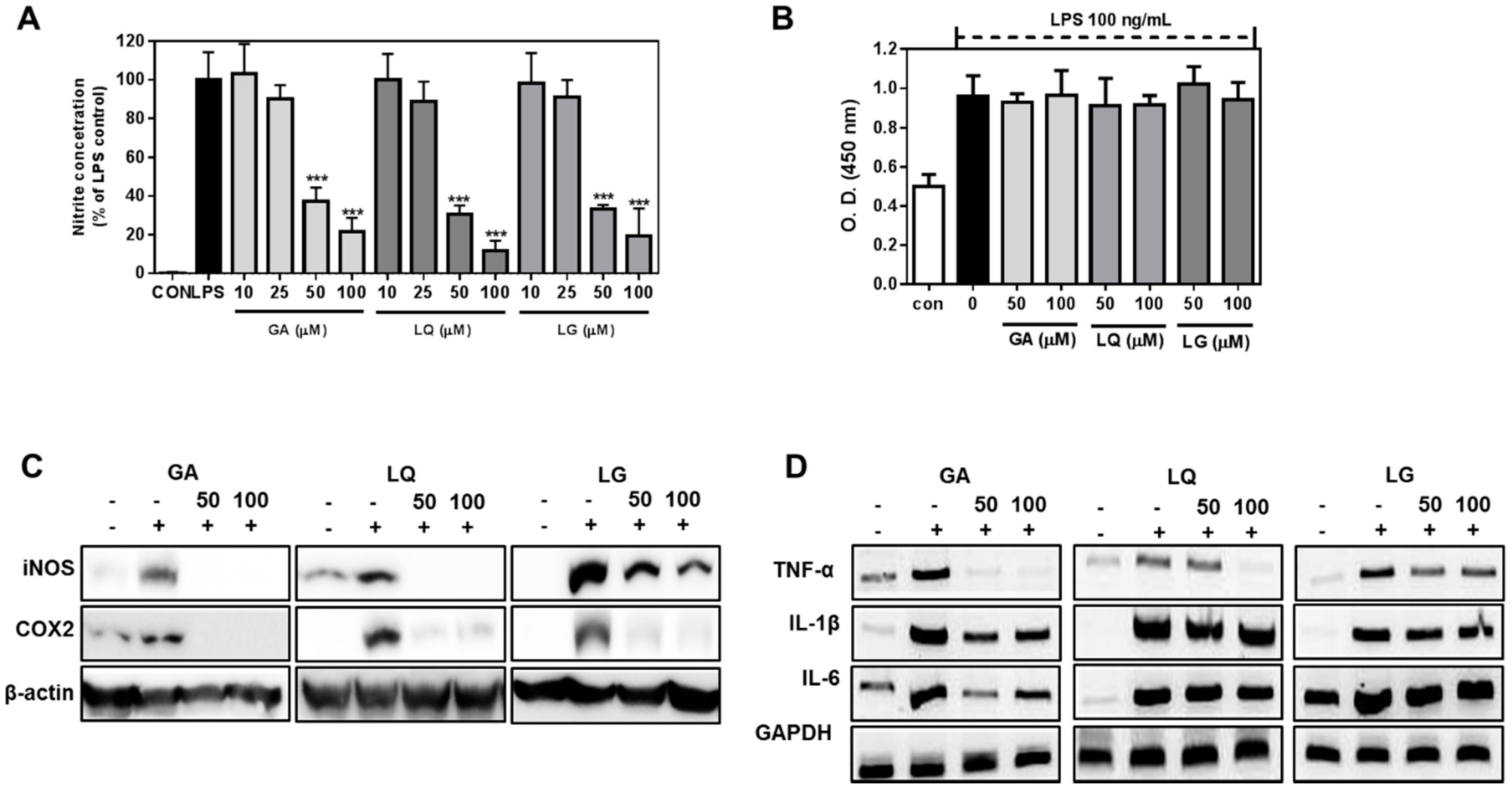
2.4. GA, LQ and LG Inhibited NO Production and Decreased the Expression Levels of iNOS and COX-2
2.5. Hepatoprotective Effect of Licorice Extract on the t-BHP-Induced Damage in Mice Liver

3. Experimental Section
3.1. BV2 Microglial Cell Culture
3.2. Chemicals and Reagents
3.3. The Original Material
3.4. Extraction and Isolation
3.5. Analysis of HPLC-MS Instrument and Conditions
3.6. Animals and Treatment
3.7. 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity
3.8. Cell Viability Assay
3.9. Nitrite Assay
3.10. Western Blot Analysis
3.11. RNA Purification and Quantitative RT-PCR
| Gene | Accession No. | Sequences | Product Size (bp) |
|---|---|---|---|
| TNF-α | NM_012675.3 | 5′-CTGGCGTGTTCATCCGTTCT-3′ 5′-GACATTCCGGGATCCAGTGA-3′ | 283 |
| IL-1β | NM_031512.2 | 5′-GAAGAGCCCGTCCTCTGTGA-3′ 5′-GTGGGTGTGCCGTCTTTCAT-3′ | 290 |
| IL-6 | NM_012589.2 | 5′-CCCACCAGGAACGAAAGTCA-3′ 5′-TCAGAATTGCCATTGCACAAC-3′ | 270 |
| GAPDH | NM_017008.4 | 5′-ATGGTGAAGGTCGGTGTGAAC-3′ 5′-TGTAGTTGAGGTCAATGAAGG-3′ | 150 |
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nencini, C.; Giorgi, G.; Micheli, L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 2007, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.; Sundari, J.S.; Balamurugan, E.; Menon, V.P. Prevention of nicotine and streptozotocin treatment induced circulatory oxidative stress by bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in diabetic rats. Mol. Cell. Biochem. 2009, 331, 1271–133. [Google Scholar] [CrossRef] [PubMed]
- Rumyana, S.; Magdalena, K.; Vessela, V.; M., M. Some In Vitro/In Vivo Chemically-Induced Experimental Models of Liver Oxidative Stress in Rats. Biomed. Res. Inter. 2014, 2014. [Google Scholar] [CrossRef]
- Gumpricht, E.; Dahl, R.; Devereaux, M.W.; Ronald, J.S. Licorice compounds glycyrrhizin and 18β-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J. Biol. Chem. 2005, 280, 10556–10563. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Horiuchi, M.; Umano, K.; Takayuki, S. Antioxidant and antiinflammatory activities of water distillate and its dichloromethane extract from licorice root (Glycyrrhiza uralensis) and chemical composition of dichloromethane extract. J. Sci. Food Agric. 2008, 88, 1158–1165. [Google Scholar] [CrossRef]
- Takhshid, M.A.; Mehrabani, D.; Ai, J.; Zarepoor, M. The healing effect of licorice extract in acetic acid-induced ulcerative colitis in rat model. Comp. Clin. Pathol. 2012, 21, 1139–1144. [Google Scholar] [CrossRef]
- Rahnama, M.; Mehrabani, D.; Japoni, S.; Edjtehadi, M.; Firoozi, M.S. The healing effect of licorice (Glycyrrhiza glabra) on Helicobacter pylori infected peptic ulcers. J. Res. Med. Sci. 2013, 18, 532–533. [Google Scholar] [PubMed]
- Paola, M.; Mariateresa, M.; Mariateresa, R.; Santo, P.; Sonia, P.; Cosimo, P. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar]
- Mohamed, A.F.; Andrea, P.; Ludger, A.W. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar]
- Cantelli-Forti, G.; Maffei, F.; Hrelia, R.; Bugamelli, F.; Bernardi, M.; Intino, R.D.; Maranesi, M.; Raggi, M.A. Interaction of Licorice on Glycyrrhizin Pharrmacokinetics. Environ. Health. Perspect. 1994, 102, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lim, J.; Bang, Y.; Gal, J.; Lee, S.U.; Cho, Y.C.; Yoon, G.; Kang, B.Y.; Cheon, S.H.; Choi, H.J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: Therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012, 23, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Shim, J.; Lee, S.; Kim, J.; Lim, S.S.; Lee, K.W.; Lee, H.J. Licorice-derived dehydroglyasperin C increases MKP-1 expression and suppresses inflammation-mediated neurodegeneration. Neurochem. Inter. 2013, 63, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez-Mateos, A.D.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailialility, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; Yu, J.Y.; Jang, Y.O.; Kim, B.T.; Han, K.J.; Jeon, Y.M.; Lee, J.C. A phenolic acid phenethyl urea compound inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines in cell culture. Inter. Immunopharmacol. 2010, 10, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.R. Functional foods. Cancer Lett. 1997, 114, 59–63. [Google Scholar] [CrossRef]
- Van Poppel, G.; van den Berg, H. Vitamins and cancer. Cancer Lett. 1997, 114, 195–202. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, K.; Wan, J.; Li, L.; Jiang, R.; Jia, M.; Jing, Y.; Zhang, L. Aminotriazole attenuated carbon tetrachloride-induced oxidative liver injury in mice. Food Chem. Toxicol. 2012, 50, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Upur, H.; Amat, N.; Blazekovic, B.; Talip, A. Protective effect of Cichorium glandulosum root extract on carbon tetrachloride-induced and galactosamine-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, C.H.; Lee, H.S.; Kim, S.T.; Sohn, U.D.; Park, E.S.; Bang, J.S.; Lee, J.H.; Chung, Y.H.; Jeong, J.H. The role of phosphatidylcholine and deoxycholic acid in inflammation. Life Sci. 2014, 108, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Melih, A.; Savas, D.; Selma, A.Y.; Kubilay, G.; Emine, T.; Erkan, T.; Rauf, T.A.; Aysun, B.; Mehmet, N.E. Attenuation of ureteral obstruction-induced renal injury by polyenylphosphatidylcholine. Inter. J. Urol. 2007, 14, 350–356. [Google Scholar]
- Jeong, H.G.; You, H.J.; Park, S.J.; Moon, A.R.; Chung, Y.C.; Kang, S.K.; Chun, H.K. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P4502E1 expression. Pharmacol. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydoperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; Wang, C.J.; Chou, F.P.; Tseng, T.H.; Hsieh, Y.S.; Lin, W.L.; Chu, C.Y. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch. Toxicol. 2002, 76, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Wang, J.M.; Chu, C.Y.; Cheng, M.T.; Tseng, T.H. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 2002, 40, 635–641. [Google Scholar] [CrossRef]
- Munhoz, C.D.; García-Bueno, B.; Madrigal, J.L.; Lepsch, L.B.; Scavone, C.; Leza, J.C. Stressinduced neuroinflammation: Mechanisms and new pharmacological targets. J. Med. Biol. Res. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Hesslinger, C.; Strub, A.; Boer, R.; Ulrich, W.R.; Lehner, M.D.; Braun, C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem. Soc. Trans. 2009, 37, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, S. COX-2 as a novel target of CRF family peptides participating in inflammation. Biochem. Biophys. Res. Commun. 2009, 382, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Weissman, B.A.; Gross, S.S. Measurement of NO and NO synthase. Curr. Prot. Neurosci. 2001, 7. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds licorice extract are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-Y.; Ha, J.Y.; Kim, K.-M.; Jung, Y.-S.; Jung, J.-C.; Oh, S. Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver. Molecules 2015, 20, 13041-13054. https://doi.org/10.3390/molecules200713041
Yu J-Y, Ha JY, Kim K-M, Jung Y-S, Jung J-C, Oh S. Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver. Molecules. 2015; 20(7):13041-13054. https://doi.org/10.3390/molecules200713041
Chicago/Turabian StyleYu, Ji-Yeon, Jae Yeo Ha, Kyung-Mi Kim, Young-Suk Jung, Jae-Chul Jung, and Seikwan Oh. 2015. "Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver" Molecules 20, no. 7: 13041-13054. https://doi.org/10.3390/molecules200713041





