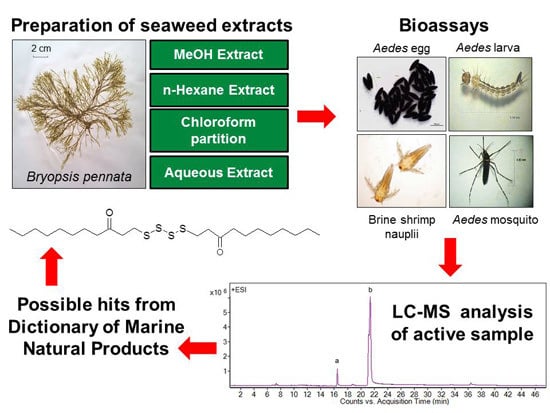
2.3. Mosquito Larvicidal Assay
The larvicidal activity of
B. pennata towards larvae of
Ae. aegypti and
Ae. albopictus at 24 h post-treatment was studied. The data clearly revealed that only chloroform extract had LC
50 values below the concentration of 100 µg/mL (
Table 2).
Table 2.
Larvicidal activity of Bryopsis pennata extracts against Aedes aegypti and Aedes albopictus.
Table 2.
Larvicidal activity of Bryopsis pennata extracts against Aedes aegypti and Aedes albopictus.
| Mosquito | Extract | LC50 (µg/mL) (95% CL) | Slope (±SE) | X2 |
|---|
| Aedes aegypti | n-Hexane | 912.86 (821.04–1095.18) | 5.25 (0.85) | 1.12 |
| Chloroform * | 92.72 (82.40–102.85) | 3.01 (0.31) | 3.14 |
| Methanol | 156.97 (133.54–179.46) | 2.57 (0.25) | 1.47 |
| Aqueous | 591.77 (528.19–692.70) | 3.78 (0.49) | 0.21 |
| Abate® 1.1G | 0.07 (0.06–0.08) | 3.21 (0.21) | 0.90 |
| Aedes albopictus | n-Hexane | 1209.50 (1123.50–1318.65) | 3.15 (0.68) | 1.83 |
| Chloroform * | 99.85 (88.68–111.27) | 2.81 (0.31) | 0.65 |
| Methanol | 177.50 (156.41–198.68) | 3.05 (0.27) | 4.02 |
| Aqueous | 692.45 (657.01–735.71) | 7.39 (0.84) | 0.70 |
| Abate® 1.1G | 0.93 (0.82–1.07) | 2.85 (0.34) | 1.72 |
As the most active extract of
B. pennata in larvicidal assay, the chloroform extract was fractioned into 8 fractions and these fractions were subjected to mosquito larvicidal assay. Out of them, A7 was the strongest larvicidal fraction with approximately 5 to 68-fold stronger activity than others (
Table 3).
Table 3.
Larvicidal activity of the fractions derived from chloroform extract of Bryopsis pennata against Aedes aegypti and Aedes albopictus.
Table 3.
Larvicidal activity of the fractions derived from chloroform extract of Bryopsis pennata against Aedes aegypti and Aedes albopictus.
| Mosquito | Fraction | LC50 (µg/mL) (95% CL) | Slope (±SE) | X2 |
|---|
| Aedes aegypti | A1 | 324.50 (308.55–356.20) | 2.68 (0.75) | 0.96 |
| A2 | 226.10 (201.50–251.35) | 2.45 (0.60) | 0.95 |
| A3 | 189.33 (160.30–210.30) | 2.86 (0.47) | 0.94 |
| A4 | 209.45 (192.34–225.19) | 2.13 (0.93) | 0.91 |
| A5 | 146.43 (123.59–167.98) | 2.21 (0.35) | 0.96 |
| A6 | 26.81 (16.53–43.47) | 1.18 (0.48) | 0.48 |
| A7 * | 4.65 (3.08–7.01) | 1.29 (0.55) | 0.68 |
| A8 | 254.29 (271.50–226.30) | 2.11 (0.67) | 0.96 |
| Abate® 1.1G | 0.07 (0.06–0.08) | 3.21 (0.21) | 0.90 |
| Aedes albopictus | A1 | 256 (239.15–281.50) | 2.11 (0.78) | 0.90 |
| A2 | 227.80 (201.45–256.87) | 2.62 (0.82) | 0.98 |
| A3 | 181.55 (165.39–201.45) | 1.88 (0.62) | 0.96 |
| A4 | 193.45 (213.90–178.32) | 1.89 (0.70) | 0.95 |
| A5 | 128.40 (108.75–152.50) | 1.73 (0.68) | 0.98 |
| A6 | 38.78 (33.89–44.37) | 3.98 (0.75) | 0.88 |
| A7 * | 5.32 (3.95–7.16) | 1.87 (0.62) | 0.81 |
| A8 | 278.90 (291.15–256.21) | 1.99 (0.74) | 0.90 |
| Abate® 1.1G | 0.93 (0.82–1.07) | 2.85 (0.34) | 1.72 |
Larvae treated with chloroform extract of B. pennata were observed to exhibit abnormal behaviour. These mosquito larvae showed signs of unnatural restlessness, wriggling movement and frequent sinking followed by floating, after 1–5 h of treatment. Such behaviour persisted until the larvae became sluggish, paralyzed and eventually sank to the bottom of the container. Mortality of the larvae was found to be on the rise from 5–20 h. Similar observations were noted for all larvae treated with different extracts except for the time and duration of exhibiting the intoxicated symptoms.
In addition, larvae under the treatment of chloroform extract of
B. pennata were observed to have darkened body segments and shrunken anal papillae as compared to the normal larvae. Further investigation under the electron microscope revealed that the treated larvae had spiracular apparatus with damaged inner structures (
Figure 1). Similar observations were noted for all larvae treated with different extracts.
Figure 1.
Photographs of Aedes albopictus larvae: (A) larva of negative control; (B) larva treated with chloroform extract of Bryopsis pennata showing darkened body parts and anal papillae; (C) larva of negative control showing intact spiracular apparatus; (D) larva treated with chloroform extract of B. pennata showing spiracular apparatus with damaged inner structures (*). ap, anal papillae; apl, anterior perispiracular lobe; pl, perispiracular lobes; ppl, posterior perispiracular lobe; s, siphon; ts, terminal spiracle.
Figure 1.
Photographs of Aedes albopictus larvae: (A) larva of negative control; (B) larva treated with chloroform extract of Bryopsis pennata showing darkened body parts and anal papillae; (C) larva of negative control showing intact spiracular apparatus; (D) larva treated with chloroform extract of B. pennata showing spiracular apparatus with damaged inner structures (*). ap, anal papillae; apl, anterior perispiracular lobe; pl, perispiracular lobes; ppl, posterior perispiracular lobe; s, siphon; ts, terminal spiracle.
2.7. Liquid Chromatography-Mass Spectrometry Analysis
Since chloroform extract and A7 fraction of
B. pennata exhibited strong larvicidal activity, their chemical constituents were investigated and analyzed by using liquid chromatography-mass spectrometry (LC-MS). The LC-MS profile of the chloroform extract exhibited 17 peaks that were resolved in 36 min (
Figure 2A), while the LC-MS profile of A7 fraction showed two main peaks (
i and
ii) (
Figure 2B).
Figure 2.
LC-MS extracted ion chromatogram of Bryopsis pennata, (A) chloroform extract and (B) A7 fraction.
Figure 2.
LC-MS extracted ion chromatogram of Bryopsis pennata, (A) chloroform extract and (B) A7 fraction.
All the main peaks belonging to various compounds were tentatively assigned by comparing the data acquired by Mass Hunter Acquisition Data to Dictionary of Natural Products and Dictionary of Marine Natural Products (
Table 7). Based on the comparison, peak
13 was tentatively assigned as an unbranched alkenic ketone, peaks
2 and
5 as alkaloids, peaks
3,
6 and
11 as steroids, peak
4 as a meroterpenoid, peaks
9,
10 and
17 as diterpenoids, peaks
14 and
16 as sesquiterpenoids, and peak
8 as a triterpenoid. The remaining four peaks did not match any data in the above mentioned dictionaries (peak
1,
7,
12 and
15). Peak
i (at 16.4 min) in A7 fraction and peak
12 (at 16.3 min) in the chloroform extract shared the same accurate mass at 768.4909; while peak
ii (at 21.6 min) in A7 fraction corresponded to peak
13 (at 21.7 min) [assigned as bis-(3-oxoundecyl) tetrasulfide] in the chloroform extract, yielding an accurate mass of 466.3487. Peaks that shared similar retention times and accurate masses were suggested to be the same compound.
Table 7.
LC-MS analysis and database search of main peaks in chloroform extract of Bryopsis pennata (Bryopsidales: Bryopsidaceae).
Table 7.
LC-MS analysis and database search of main peaks in chloroform extract of Bryopsis pennata (Bryopsidales: Bryopsidaceae).
| No | Rt (min) | Accurate Mass | Possible Molecular Formula | Possible Hits from Database | Type of Compound and Biological Source (Order: Family) |
|---|
| 1 | 8.4 | 326.1923 | C19H34O4 | No hits | |
| 2 | 8.8 | 370.2187 | C22H14N2O4 | Caulerpinic acid | Alkaloid of green seaweed Caulerpa racemosa (Bryopsidales: Caulerpaceae) |
| 3 | 9.1 | 414.2442 | C29H50O | Sitosterol | Steroid of green seaweed Bryopsis plumose (Bryopsidales: Bryopsidaceae) |
| 4 | 9.4 | 340.2079 | C16H21BrO3 | Hydroxycymopochromenol | Meroterpenoid of green seaweed Cymopolia barbata (Dasycladales: Dasycladaceae) |
| 5 | 9.7 | 384.2342 | C23H16N2O4 | Monomethyl caulerpinate | Alkaloid of green seaweed Caulerpa racemosa (Bryopsidales: Caulerpaceae) |
| 6 | 10.0 | 428.2604 | C29H48O2 | Decortinol | Steroid of green seaweeds Codium decorticatum and Codium arabicum(Bryopsidales: Codiaceae) |
| 7 | 10.2 | 472.2874 | C29H44O5 | No hits | |
| 8 | 10.4 | 516.3135 | C34H60O3 | Botryolin A and B | Triterpenoid of microalga Botryococcus braunii (Trebouxiales: Botryococcaceae) |
| 9 | 10.8 | 302.183 | C20H30O2 | 2,6,10,14-Phytatetraene-1,20-dial | Diterpenoid of green seaweed Caulerpa brownie (Bryopsidales: Caulerpaceae) |
| 10 | 11.1 | 346.2087 | C22H34O3 | 2,6,10,14-Phytatetraene-1,20-diol, Variant: (2E,6E,10E)-form, Derivative: 1-Aldehyde, 20-Ac | Diterpenoid of green seaweed Caulerpa brownie (Bryopsidales: Caulerpaceae) |
| 11 | 15.8 | 444.2243 | C29H48O3 | 7-Hydroperoxystigmasta-5,25-dien-3-ol | Steroid of green seaweed Codium arabicum (Bryopsidales: Codiaceae) |
| 12 | 16.3 | 768.4909 | C43H60O12 | No hits | |
| 13 | 21.7 | 466.3487 | C22H42O2S4 | Bis-(3-oxoundecyl) tetrasulfide * | Unbranched alkenic ketone of brown seaweed Dictyopteris spp.(Dictyotales: Dictyotaceae) |
| 14 | 23.0 | 278.1494 | C17H26O3 | 4-Hydroxy-2-[2-(2,6,6-trimethyl-2-cyclohexen-1-yl)ethyl]-2-buten-1-al, Derivative: Ac | Sesquiterpenoid of green seaweed Caulerpa flexilis (Bryopsidales: Caulerpaceae) |
| 15 | 24.4 | 921.0025 | C54H83NO11 | No hits | |
| 16 | 34.9 | 390.2742 | C21H26O7 | 10,11-Epoxycaulerpenyne | Sesquiterpenoid of green seaweed Caulerpa taxifolia (Bryopsidales: Caulerpaceae) |
| 17 | 35.4 | 390.2749 | C24H38O4 | Trifarin | Diterpenoid of green seaweed Caulerpa flexilis (Bryopsidales: Caulerpaceae) |
2.8. Discussion
Development of new insecticides based on natural products requires a thorough understanding of the potential activity of natural products against mosquito at each stage of the insect’s life. Furthermore, the information on toxic effect of bioinsecticides against non-target organisms serves a useful basis for the development of safer and more selective mosquitocidal agents. In this study, dengue vectors of Ae. aegypti and Ae. albopictus were used as a model system and brine shrimp nauplii was used as non-target organism, to investigate the potential of different extracts of seaweed B. pennata.
Applications of ovicide and larvicide are effective strategies to control the population of mosquito since controlling the egg and larva that live in bounded aquatic area is easier compared to targeting the free-flying adult [
19]. Furthermore, as dengue virus is transmitted transovarially by mosquito, ovicide could be the solution to suppress the vector population. Therefore, inhibiting the egg hatchability, larval emergence, and oviposition of gravid female are the main aims of ovicidal agent in mosquito control. Previous studies showed that treatment of plant extract induces morphometric changes to the mosquito egg that inhibits the development of the egg, such as swelling with increase in length of the egg and deformities in air floats [
20]. Furthermore, studies also revealed that plants had promising ovicidal and oviposition repellent effects against
Ae. aegypti [
21,
22,
23]. However, ovicidal and oviposition repellent potentials of seaweeds have not been studied much. Interestingly, the ovicidal and oviposition repellent properties of chloroform and methanol extracts of
B. pennata in our report are comparable to that of other reports [
21,
24].
Earlier studies have suggested that seaweeds also exhibit skin repellent and smoke repellent properties against adult mosquitoes [
25,
26], but little information about the adulticidal properties of seaweed. In our report, chloroform extract of
B. pennata resulted in the strongest adulticidal effect among the extracts tested, but it was considered as an ineffective adulticidal agent against
Ae. aegypti when compared to extract of monocot flowering plant
Acorus calamus (LC
50 value of 0.04 mg/cm
2) and essential oil of wild sage
Lantana camara (LC
50 value of 0.06 mg/cm
2) [
27,
28]. On the other hand, the mosquitocidal activities of bioinsecticides are comprised of both toxic and behavioural effects [
29]. In the present study, the observation of abnormal behaviour changes of adult females after treatment of seaweed extract is in agreement with previous studies using other plant extracts [
28,
30] and these symptoms were also similar to those caused by nerve poison [
31].
The use of seaweeds as effective mosquito larvicide has been reported by researchers [
18,
32]. Furthermore, Bianco
et al. [
17] reported that hexane extract of red seaweed
Laurencia dendroidea had strong larvicidal effect by causing 100% mortality at 50 ppm against
Ae. aegypti larvae. Sequential fractionation of the hexane extract of
L. dendroidea yielded elatol that exhibited LC
50 value of 10.7 ppm against
Ae. aegypti larvae. This demonstrates that pure compound has higher efficiency in larvicidal activity when separated from the extract (with a combination of compounds), due to a higher concentration of the compound being available for bioactivity action. Our findings are in line with the finding of Bianco
et al. [
17] showing A7 fraction of
B. pennata exhibited more than 15-fold stronger larvicidal effect as compared to the extracts. In addition, the larvicidal activity of A7 fraction in the present study is comparable to other larvicidal compounds derived from either terrestrial plants or seaweeds [
18,
33,
34].
Apart from that, β-sitosterol (the anomer of compound which assigned as peak
3 in the LC-MS analysis of chloroform extract), was reported as an active mosquito larvicidal compound isolated from the petroleum ether extract of shrub
Abutilon indicum in a previous report with the LC
50 value of 11.49 ppm against
Ae. aegypti [
35]. However, the presence of sitosterol in the present study did not have a significant effect on the larvicidal action as compared to the previous study. The bioactivity of plant extracts and fractions depends on the biomass production and chemical composition which highly related to the natural variability and sample preparation [
18,
36]. Therefore, it is suggested that sitosterol may have a lower concentration in the extracts of
B. pennata due to different species and extraction methods; hence the weak larvicidal effect on mosquitos in the present study.
In view of the active larvicidal activity of the A7 fraction, the compounds present in the A7 fraction were assumed to correlate with the strong mosquito larvicidal effect. The match of peak
13 [assigned as bis-(3-oxoundecyl) tetrasulfide] or
ii of LC-MS analysis in the dictionary suggested that the compound has an unbranched long hydrocarbon chain with carbonyl group (
Figure 3). This is in line with the previous reports that described active mosquito larvicidal compounds with their chemical characteristics such as lipophilic profile [
37] and possession of double bond [
38]. For example, aliphatic fatty acids with a long hydrocarbon chain derived from green seaweed
Cladophora glomerata (having LC
50 values of 3‒14 ppm against
Aedes triseriatus) [
39] and alkaloids with double bond isolated from green seaweed
Caulerpa racemosa (having LC
50 values of 1.4‒4.8 ppm against
Culex pipiens) [
40]. Further confirmation of the identity of peak
13 or
ii and its larvicidal activity is warranted, as the literature on bis-(3-oxoundecyl) tetrasulfide is limited [
41,
42].
Figure 3.
Bis-(3-oxoundecyl) tetrasulfide (possible hit of Peak 13 or ii from Dictionary of Marine Natural Products).
Figure 3.
Bis-(3-oxoundecyl) tetrasulfide (possible hit of Peak 13 or ii from Dictionary of Marine Natural Products).
In spite of exhibiting killing action towards mosquito at different stages of its life cycle, seaweed has been proven to have deleterious impact on the morphological structure and behaviour of the treated mosquitoes [
17,
32]. These symptoms were noted in our observation of the study. Structural alteration of anal papillae of mosquito larvae leads to its dysfunctionality which may result an interruption of osmosis and ionic regulations [
43,
44]. Osmosis and ionic imbalance of the mosquito larvae may be intrinsically associated with the larval death or may be part of the mechanism causing the death of mosquito larvae [
45]. Furthermore, the rupture of larva’s inner structure of spiracular apparatus observed in the present report is suggested to cause destruction to the hydrophobic surface of stigmal plate, causing water/medium to enter the tracheal trunk which harms the respiration system of the larvae [
46,
47]. On the other hand, the abnormal behaviour of intoxicated larvae might be due to the effect of insecticidal extracts that affects the neuromuscular coordination in chemical synapses [
48]. In addition, interaction with the picrotoxinin receptor of insect central nervous system (cyclodiene-type mechanism) is proposed to be one of the action modes of insecticidal compounds derived from seaweeds [
49].
An effective mosquitocidal agent should be target-specific but pose little risk to the non-target organism. Therefore, the brine shrimp nauplii toxicity test offers a relatively rapid and convenient method to assess the toxic effect of natural products [
50]. Various seaweeds have been tested for their toxicity against brine shrimp nauplii [
18]. In our report,
B. pennata induced strong larvicidal effect towards
Aedes mosquito but weak toxic effect towards brine shrimp nauplii. A similar trend was observed in the study of brown seaweed
Padina gymnospora reported by Guedes
et al. [
51].
To the best of our knowledge, this is the first report on the mosquitocidal properties of green seaweed B. pennata against dengue vectors Ae. aegypti and Ae. albopictus. Although the present study demonstrated the mosquitocidal potential of B. pennata, identification of active compounds and their mechanism of action, as well as their possible synergistic effect, may allow the development of bioinsecticides with greater potency than the extract and fraction evaluated here. Chemical synthesis of the active larvicidal analogues and formulation of binary insecticides may also provide useful end products. In addition, the insecticide should be tested on different mosquito species and in the field to ensure its efficacy.