Abstract
Three new hydrazide and five new hydrazonoyl derivatives were synthesized. The chemical structures of these compounds were confirmed by 1H-NMR, IR spectroscopy and elemental analysis. The prepared compounds were tested for their activity to inhibit photosynthetic electron transport in spinach chloroplasts and growth of the green algae Chlorella vulgaris. IC50 values of these compounds varied in wide range, from a strong to no inhibitory effect. EPR spectroscopy showed that the active compounds interfered with intermediates Z•/D•, which are localized on the donor side of photosystem II. Fluorescence spectroscopy suggested that the mechanism of inhibitory action of the prepared compounds possibly involves interactions with aromatic amino acids present in photosynthetic proteins.
1. Introduction
Hydrazides, hydrazonoyl halides and hydrazonoyl cyanides exhibit a wide spectrum of biological activities including antimicrobial [1,2,3,4,5,6,7], antifungal [8,9], antibacterial [8,10], antituberculotic [5,11], anticancer, anti-inflammatory and analgesic effects [12]. The biological activities and applications of hydrazone derivatives were comprehensively reviewed by Rollas and Küçükgüzel [13], Kumar and Narasimhan [14] and Narang et al. [15]. Some hydrazine derivatives induced DNA fragmentation [16] and exhibited mutagenic activity [17,18]. Hydrazides of aromatic aldehydes were found to be phytotoxic with specificity for Amaranthus retroflexus L. by absorption through the foliage [19]. N′-(4-methylphenylsulfonyl)-3-bromothiophene-2-carbohydrazonoyl chloride and 3-(4-benzyl-1-yl)-1-(toluene-4-sulfonyl)-1H-thieno[3,2-c]pyrazole were synthesized as dopamine D3 receptors [20]. Maleic hydrazide has been used in agriculture as a major commercial herbicide since 1950, however, later studies showed that it exhibits mutagenic effects [21,22].
Hydrazine is an electron donor to the oxidizing side of photosystem II (PSII) and in photosynthesis it supports a light-dependent electron flow in chloroplasts inhibited at the water-oxidizing complex (WOC) [23]. Treatment of thylakoids with hydrazine permits a high population of the redox states S0, S−1, and S−2 in the water oxidase, a complex enzyme which integrates a photochemical reaction centre, PSII, and a catalytic centre, a manganese cluster [24]. According to Förster and Junge [25], two bridging ligands, possible Cl− or OH−, which normally connect two Mn nuclei, can be substituted by two molecules of hydrazine when the WOC resides in state S1. The reactivity of hydrazine with PSII depends strongly on redox state of WOC [26]. Treatment of chloroplasts with high hydrazine concentration (1 mmol/dm3) resulted in complete inhibition of water splitting reaction under flash light [27]. The dichlorophenol-indophenol (DCPIP) reduction by PSII in chloroplasts prepared from leaves of Phaseolus vulgaris L. was significantly decreased by maleic hydrazide treatment, while ferricyanide reduction activity was significantly accelerated and it was assumed that the site of action of maleic hydrazide is not situated in PSII but it is between cytochrome and plastocyanin on the donor side of PSI [28]. N′-phtalohydrazine and dichlorophenylhydrazine were found to be relatively efficient donors to PSII, although less efficient than hydrazobenzene [29]. The photosynthetic electron transpot (PET) inhibiting activity of hydrazones derived from furo[3,2-b]pyrrole-5-carboxhydrazides by their reactions with substituted furan-2-carboxaldehydes or thiophene-2-carboxaldehyde varied in the range from 0.071 to 2.060 mmol/dm3 and application of these compounds at concentrations 1–100 μmol/dm3 mostly did not affect significantly chlorophyll content in Chlorella vulgaris [30]. On the other hand, mixtures containing hydrazone compounds and copper were patented as suitable for controlling the growth of algae [31]. The ionophore carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone acts as an uncoupler of ATP-ase, because it disrupts proton transport-coupled ATP synthesis [32,33].
The synthesis of novel hydrazone derivatives is perspective because of their potential use as antimicrobial or therapeutical compounds. In our current work we prepared three new derivatives of hydrazide and five hydrazonoyl derivatives and analyzed their inhibitory effect on PET in spinach chloroplasts by Hill reaction, fluorescence and EPR spectroscopy.
2. Results and Discussion
2.1. Chemistry
The starting compounds for the synthesis of organic hydrazonoyl chlorides 3b, 3c, 3d, 3e and one hydrazonoyl cyanide 4f, were carbohydrazides and acethydrazide 1a–1c. These hydrazides were prepared in high yields by nucleophilic substitution reactions of the corresponding methyl esters with hydrazine hydrate in propanol at 80 °C for 10–12 h as described in [34,35,36,37]. N-substituted hydrazides 2a, 2b, 2c were prepared by nucleophilic substitution reactions of 1-chloro-4-trifluoromethyl-2,6-dinitrobenzene and the corresponding hydrazides 1 (Scheme 1) in anhydrous dimethoxyethane with triethylamine as catalyst, for 2–3 h at room temperature [38,39,40]. The yields of these products were 74%–80%, after recrystallization.
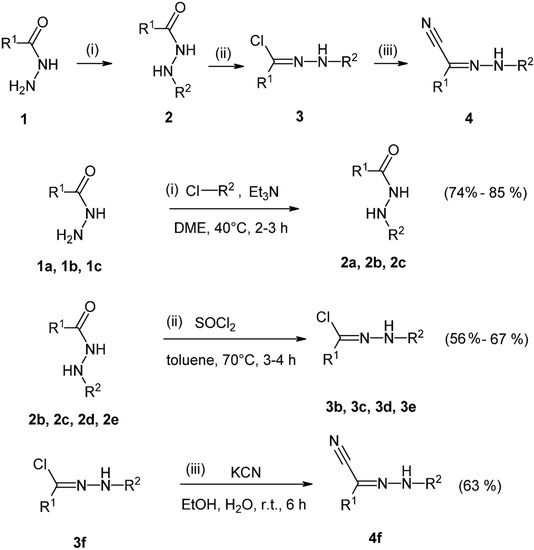
Scheme 1.
Preparation of N′-substituted hydrazides 2 (2a, 2b, 2c), N′-substituted hydrazonoyl chlorides 3 (3b, 3c, 3d, 3e) and N′-substituted hydrazonoyl cyanide 4 (4f); (i), (ii), (iii), reactants and reaction conditions; 1a, 2a, R1 = 4-methoxyphenyl, 1b, 2b, 3b, R1 = 4-tert-butylphenyl, 1c, 2c, 3c, R1 = methyl; 2d, 3d, R1 = 4-fluorophenyl, 2e, 3e, R1 = thiophene-2-yl, 3f, 4f, R1 = naphtalene-2-yl; R2 = 2,6-dinitro-4-(trifluoromethyl)phenyl, for compounds 2, 3 and 4. Yields of products (%) are indicated in brackets. Compounds 1a, 1b and 1c were prepared according to literature [34,35,36,37] and compounds 2e, 2d, and 3f were obtained from Tauchem (Bratislava, Slovakia).
Hydrazonoyl chlorides 3b, 3c, 3d and 3e were prepared by nucleophilic substitution reaction of N′-substituted hydrazides 2a, 2b and 2c and thionyl chloride [40,41,42,43]. The reaction (Scheme 1) was carried out for 3–4 h in toluene, at 70 °C. The yields of products were 56%–67% after purification. Hydrazonoyl cyanide 4f was prepared from corresponding hydrazonoyl chloride as starting compound and potassium cyanide in an ethanol–water mixture [40]. The yield was 63% after recrystallization from cyclohexane. All prepared compounds were solid compounds. Hydrazonoyl chlorides and hydrazonoyl cyanide were colored. Structure of all newly prepared compounds was confirmed by 1H-NMR, IR spectroscopy and elemental analysis.
2.2. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
Five of the studied compounds inhibited PET in spinach chloroplasts. However, their inhibitory activity varied over a wide range. The IC50 values of the studied compounds are presented in Table 1 (second column). The most effective derivative was N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]thiophene-2-carbohydrazonoyl chloride (3e) with an IC50 = 2.34 μmol/dm3, which is comparable to the classical herbicide diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea; DCMU) with an IC50 = 1.9 μmol/dm3 [44]. The high activity of compound 3e may be associated with the presence of the thiophene nucleus in this compound because the free electron pair of the sulphur in the thiophene moiety could interact with constituents of the photosynthetic apparatus via hydrogen bonds. On the other hand, no PET inhibition was observed for compounds 2a, 2c and 4f. In our current study, we have chosen 4-trifluoromethyl-2,6-dinitrophenyl substitution for N′-substituted hydrazides and hydrazonoyl derivatives based on our previous observations that electron-withdrawing substituents, such as CF3, showed effective PET inhibition [45,46,47,48].

Table 1.
IC50 values (μmol/dm3) of studied hydrazide and hydrazonoyl derivatives.
| Compound | PET Inhibition in Chloroplasts | Growth Inhibition of Chlorella vulgaris |
|---|---|---|
| 2a | no inhibition | no inhibition |
| 2b | 18.0 | 25.87 |
| 2c | no inhibition | 8.01 |
| 3b | 146.0 | 17.58 |
| 3c | 2213 | 10.32 |
| 3d | 61.4 | 13.32 |
| 3e | 2.34 | 12.30 |
| 4f | no inhibition | no inhibition |
| DCMU | 1.9 * | 7.3 ** |
Values taken from the literature: *: [44], **: [49].
Next, we attempted to determine the site of action of the studied compounds by studying the emission spectra of chlorophyll in spinach chloroplasts treated with the studied compounds. The fluorescence spectra of spinach chloroplasts treated with compounds under study are presented in Figure 1. We identified the emission peak at 684 nm corresponding to Chla, located in the pigment protein complexes of the photosystem PSII and a shoulder at 739 nm which corresponds to fluorescence of Chla located in the pigment protein complexes of the PSI [50]. This experiment showed that the compounds which inhibited the PET in spinach chloroplasts quenched the fluorescence of chlorophyll molecules present in the pigment protein complexes of both photosystems. On the other hand, the compounds which did not inhibit PET (2a, 2c and 4f) increased the fluorescence of chlorophyll. This effect may be caused by defects in photoreduction of QA. A similar effect has been observed by Haveman et al. [29] on PSII particles treated with hydrazobenzene. Based on these findings, we can assume that the sites of action of the studied compounds are both photosynthetic centers PSI and PSII.
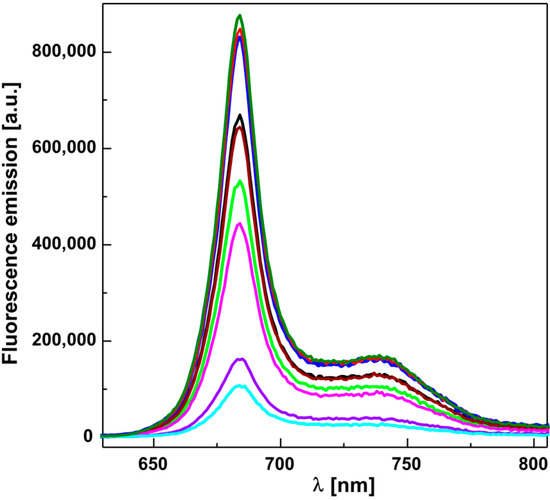
Figure 1.
Fluorescence emission spectra of untreated spinach chloroplasts and those treated with 5 μmol/dm3 of studied compounds (from top to bottom: 4f—olive; 2c—red; 2a—blue; control sample—black; 3c—wine; 3e—green; 3b—magenta; 3d—violet; 2b—cyan).
We speculated that the mechanism of inhibitory action of the studied compounds may involve their interaction with proteins present in the photosynthetic reaction centers. To test our hypothesis, we analyzed the effect of studied compounds on the fluorescence of aromatic amino acids present in spinach chloroplasts. Quenching of the fluorescence was observed when compound 3e was added to spinach chloroplasts (Figure 2). A similar effect was observed with other tested compounds (data not shown).
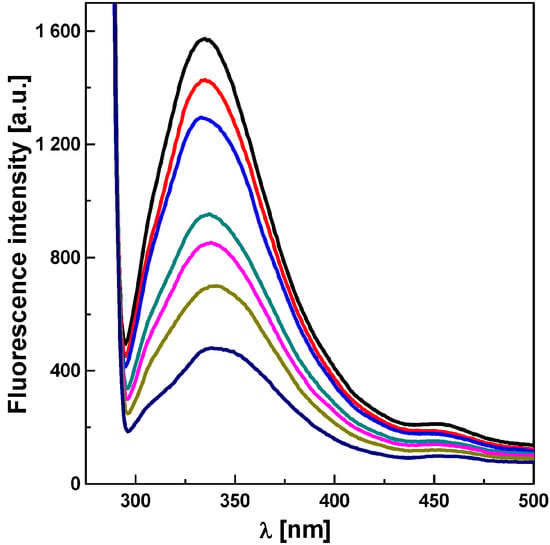
Figure 2.
Effect of N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]thiophene-2-carbohydrazonoyl chloride on fluorescence of chloroplast amino acids. Compound 3e was added to spinach chloroplasts to a final concentration of 0, 10, 20, 30, 40, 60, 80 μmol/dm3 (from top to bottom).
Using EPR experiments, we found that studied compounds inhibiting the PET immediately decreased the intensity of both EPR signals originating from the intermediates Z• and D•, alternatively, only D• (Figure 3). The EPR spectrum of the intermediate D• (g = 2.0046, ∆BPP = 2 mT), which corresponds to the radical of tyrosine at position 161 in the D2 protein of PSII [51], is shown as black line in the EPR spectrum of untreated chloroplasts in the dark (Figure 3A, black line). The increase of intensity in the EPR spectra of untreated chloroplasts in the light represents EPR signal of the intermediate Z• (Figure 3A, difference between black and red lines; g = 2.0046, ∆BPP = 2 mT), which corresponds to the radical of tyrosine situated at position 161 in the D1 protein of PSII [52]. We found that the studied compounds had different impacts on the intermediates Z•/D•. Substances that showed no inhibition of the Hill reaction (2a and 2c) exhibited very little effect on intermediates Z•/D• which resulted in only minor changes in the EPR spectra of chloroplasts treated with these compounds. EPR spectra of chloroplasts treated with compound 2a are shown in the Figure 3B. We observed a similar effect in chloroplasts treated with compound 2c (data not shown). Compounds that inhibited the Hill reaction affected differently function of intermediates Z•/D•. Almost complete disappearance of the signal corresponding to D• was observed in EPR spectra of chloroplasts treated with compounds 2b, 3b, 3c and 3d (Figure 3C, black line), whereas the signal from the intermediate Z• remained unchanged (Figure 3C red line). EPR spectra of chloroplasts treated with compound 2b are shown in the Figure 3C. We observed a similar effect in chloroplasts treated with compound 3b, 3c and 3d (data not shown). On the other hand, compounds 3e and 4f interfered with both intermediates Z•/D• which is demonstrated by the complete disappearance of both signals in the EPR spectra of chloroplasts treated with these agents (Figure 3D,E, black and red lines). One possible explanation is that the interaction of studied compounds with intermediates Z•/D• resulted in interruption of electron transfer from PSII to PSI that caused a large increase of the EPR signal (g = 2.0026, ∆BPP = 1 mT) corresponding to the core of PSI (P680+), thus oxidized form of Chla dimer [53] (Figure 3C,D, red lines). The compound 4f oxidized P680 already in the dark (Figure 3E, black line).
Interestingly, in chloroplasts in which PET was inhibited by addition of active compounds (i.e., 2b, 3b, 3c, 3d and 3e), a recovery of the PET nearly to the original levels was observed after the addition of DPC. This experiment suggests that the studied compounds may act at the donor side of PSII in the complex decomposing water or at the sites of Z•/D• intermediates. Our finding is consistent with previous findings of Heath [23] and Förster and Junge [25]. In order to determine whether PET through PSI is damaged, we carried out experiments with chloroplasts treated with the studied compounds and DCPIPH2, an artificial electron donor operating in plastocyanin on the donor side of PSI and using methyl viologen as the final artificial electron acceptor of PSI. We found that in such treated chloroplasts, with the exception of the compound 4f (which caused oxidation of P680 already in the dark), PET through PSI was not interrupted. Thus, it is likely the studied compounds have other site of action, for example, cytochrome b6f complex, located between the PSI and PSII. Interestingly, a similar conclusion was published by Haveman et al. [29], who found that hydrazobenzene, which is structurally similar to compounds synthesized in our current study, can be oxidized in at least two photoreactions. In the first reaction, it acts as an efficient donor for PSII and this reaction is inhibited by 5 μM DCMU, while a second hydrazobenzene-DCPIP reaction, which is not inhibited by DCMU, is presumably catalyzed via an oxidized component of the redox chain between the primary stable electron acceptor of PSII and the quencher of chlorophyll fluorescence and PSI.
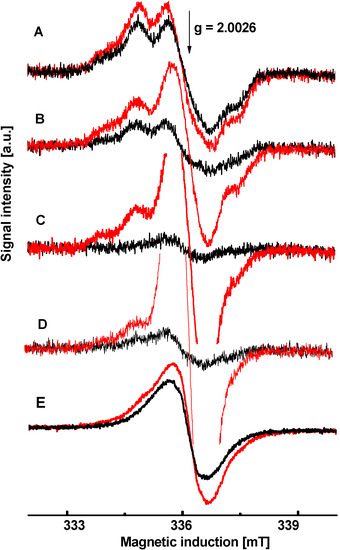
Figure 3.
EPR spectra of untreated spinach chloroplasts (A) and those treated with 0.05 mol/dm3 of 2a (B); 2b (C); 3e (D) and 4f (E). Black curves indicate spectra registered in the dark. Red curves indicate spectra registered in the light.
2.3. Inhibition of Algae Growth
To determine a long-time effect of the studied compounds on a photosynthetic organism, we analyzed the effect of these compounds on growth of the green algae Chlorella vulgaris monitoring chlorophyll concentrations in algal suspensions according to Pavlíková et al. [54]. Six compounds effectively inhibited the growth of algae (Table 1, third column). For an illustration, the antialgal activity of DCMU is about 7.3 μmol/dm3 [49]. Interestingly, the most effective compound was 2c, which did not inhibit PET in spinach chloroplasts. This suggests that the compound 2c acts in algae on targets other than photosynthetic centers, for example, it may inhibit enzymes involved in biosynthesis of chlorophyll or stimulate some other enzymes involved in its degradation.
3. Experimental Section
3.1. General Information
Ethyl acetate p.a., n-hexane p.a., propanol p.a., ethanol p.a., 2,6-dichlorophenol–indophenol (DCPIP), 1,5-diphenylcarbazide (DPC), TRIS, MgCl2, saccharose, 1,1′-dimethyl-4,4′-bipyridinium dichloride hydrate (methyl viologen), dimethoxyethane (DME), triethylamine, dimethylsulfoxide p.a. (DMSO), 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and thionyl chloride were purchased from Centralchem (Bratislava, Slovakia). 1-Chloro-4-trifluoromethyl-2,6-dinitrobenzene was purchased from Alfa Aesar (Ward Hill, MA, USA). 4-Ethoxybenzohydrazide, acethydrazide and 4-tert-butylbenzohydrazide were prepared according to literature [34,35,36,37]. N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]thiophene-2-carbohydrazide (2e), N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]-4-fluorobenzohydrazide (2d) and N′-[2,6-dinitro-4-(trifluoromethyl)]-2-naphthohydrazonoyl chloride (3f) were purchased from Tauchem (Bratislava, Slovakia).
Melting points were determined on a Kofler hot plate apparatus and are uncorrected. IR spectra were obtained on a NICOLET NEXUS 470 spectrophotometer in KBr. Elemental analyses were obtained on Elemental Analyzer Carlo Erba CHNS-OEA 1108. 1H-NMR spectra at 300 MHz were obtained on Varian Gemini 2000 spectrophotometer in DMSO-d6 with tetramethylsilane as an internal standard. The purity of prepared compounds and course of reactions were checked on Merck TLC Silica gel 60 F254 plates in ethyl acetate–n-hexane as the mobile phase.
3.2. Synthesis
3.2.1. General Procedure for Synthesis of N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]hydrazides 2a, 2b, 2c
A solution of 1-chloro-2,6-dinitro-4-(trifluoromethyl)benzene (10 mmol) in 1,2-dimethoxyethane (20 mL) was added dropwise to a stirred solution of of N′-substituted hydrazide 1a, 1b, 1c (10 mmol) and triethylamine (10 mmol) in anhydrous dimethoxyethane (40 mL) over a period of 30 min at 20–25 °C. Then reaction mixture was heated to 40 °C and stirred for 2–3 h. The reaction was monitored by TLC. The reaction mixture was poured on ice water (200 mL). The obtained solid product was filtered off, washed with water and recrystallized from 80% acetic acid.
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-4-methoxybenzohydrazide (2a)
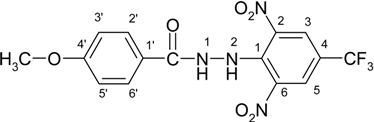
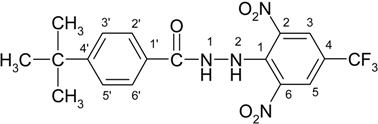
4-(tert-Butyl)-N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]benzohydrazide (2b)
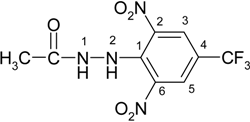
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]acethydrazide (2c)
3.2.2. General Procedure for Synthesis of N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]hydrazonoyl Chlorides 3b, 3c, 3d, 3e
Thionyl chloride (7 mmol) was added portionwise over a period of 20 min to a stirred solution (or suspension) of N′-[2,6-dinitro 4-(trifluoromethyl)phenyl] hydrazides (6 mmol) 2a, 2b, 2c, in toluene (30 mL). Then reaction mixture was heated to 70 °C over 3–4 h. The reaction was monitored by TLC. The mixture was washed with ice water, 5% aqueous solution of sodium bicarbonate (3 × 10 mL) and then with water. Organic layer was dried with anhydrous Na2SO4. The mixture was filtered and toluene was evaporated from the filtrate by distillation. The crude product was purified by recrystallization.
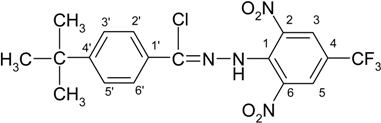
4-(tert-Butyl)-N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]benzohydrazonoyl chloride (3b)
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]acethydrazonoyl chloride (3c)
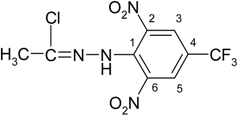
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-4-fluorobenzohydrazonoyl chloride (3d)
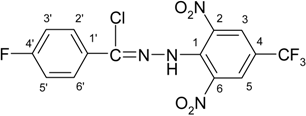
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]thiophene-2-carbohydrazonoyl chloride (3e)
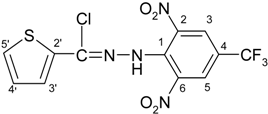
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-2-naphtohydrazonoyl cyanide (4f)
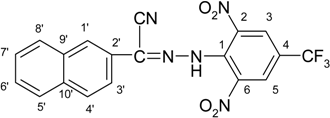
Solid N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]-2-naphtohydrazonoyl chloride was added portionwise over a period of 30 min at room temperature to a solution of potassium cyanide (0.33 g, 5 mmol) in ethanol (20 mL) and water (14 mL). After that ethanol (10 mL) and water (7 mL) were added into the reaction mixture and stirred at room temperature for 12 h. The reaction was monitored by TLC. The reaction mixture was poured into water (30 mL). The crude solid product was filtered, washed with water and vacuum-dried. The crude product was recrystallized from cyclohexane. Yield 1.08 g (63%); yellow solid, Mp 253–255 °C (cyclohexane). Anal. Calc. or C19H10F3N5O4 (429.32) C, 53.16; H, 2.35; N, 16.31. Found: C, 52.98; H, 2.46; N, 16.50%, IR 3232 (v/NH), 2216 (v/CN), 1525, 1537 (v/NO2), 1634 (v/CN). 1H-NMR δ: 11.88 (br, s, 1H, NH), 8.74 (br, s, 2H, H-3, H-5), 8.26 (br, s, 1H, H-1′), 8.14–7.98 (m, 3H, H-4′, H-5′, H-8′), 7.71–7.63 (m, 3H, H-3′, H-6′, H-7′).
3.3. PET Study
PET was monitored in spinach chloroplasts prepared according to our previous work [55]. PET through PSII was monitored by the Hill reaction with DCPIP as an artificial electron acceptor, or by using DPC as an electron donor for intermediate D in PSII [56]. PET through PSI was monitored using DCPIPH2 as an electron donor and methyl viologen as an artificial electron acceptor for PSI [56]. DCPIP photoreduction or oxidation of DCPIPH2 was determined spectrophotometrically (Genesys 6, Thermo Scientific, Waltham, MA, USA). The chlorophyll (Chl) concentration in these experiments was 30 mg/dm3. The inhibitory activities of the studied compounds were expressed by IC50 values, i.e., molar concentrations of the compounds causing 50% decrease of absorbance at 600 nm compared to control sample. The effect of the studied compounds on the growth of the green algae Chlorella vulgaris was analyzed as described in our previous work [54].
Chlorophyll fluorescence of spinach chloroplasts was recorded at room temperature by spectrofluorimeter FSP 920 (Edinburgh Instruments, Livingston, UK) using an excitation wavelength λex = 436 nm. Fluorescence of aromatic amino acids was monitored by a F-2000 spectrophotometer (Hitachi, Tokyo, Japan) using excitation wavelength λex = 275 nm, according to our previous work [57]. Both fluorescence experiments were performed in 1 cm fluorescence cell in the right-angle arrangement. The chlorophyll concentration in chloroplast suspension was 10 mg/dm3.
EPR experiments were performed by the X-band EPR spectrometer (EMX Plus, Bruker, Germany) at 5 mW microwave power and 0.5 mT modulation amplitude. The chloroplast suspensions were mixed with studied compounds directly before the EPR measurements and immediately transferred to a small quartz flat cell (WG 808-Q, optical cell length 0.04 cm; Wilmad-LabGlass, Vineland, NJ, USA). The samples were irradiated at 295 K directly in the EPR resonator through 5 cm water filter, and the EPR spectra were recorded in situ during continuous photoexcitation. The irradiation source was a 150 W halogenated lamp. The chlorophyll concentration was 4.0 g/dm3.
Due to the limited solubility of the samples, these were added to the chloroplast suspensions as a DMSO solution. DMSO at a concentration of 10% did not influence the above-mentioned photochemical reaction in the chloroplast (data not shown).
4. Conclusions
In this work we prepared three new N′-[2,6-dinitro-4-(trifluoromethyl) phenyl)]hydrazide derivatives and five N′-[2,6-dinitro-4-(trifluoromethyl)phenyl)]hydrazonoyl derivatives. Hydrazides were synthesized by nucleophilic substitution reactions of the corresponding methyl esters with hydrazine hydrate. Four hydrazonoyl chlorides were prepared by reaction of the corresponding N′-substituted hydrazides with thionyl chloride. Starting compounds for the preparation of N′-[2,6-dinitro-4-(trifluoromethyl)-phenyl]-2-naphthohydrazonoyl cyanide were N′-[2,6-dinitrophenyl-4-(trifluoromethyl)]-2-naphthohydrazonoyl chloride and potassium cyanide. The chemical structures of these compounds were confirmed by 1H-NMR, IR spectroscopy and elemental analysis. The majority of compounds exhibited inhibitory effect on photosynthesis in spinach chloroplasts and on growth of the green algae Chlorella vulgaris. The IC50 values of these compounds varied in wide range, from a strong to no inhibitory effect. EPR spectroscopy showed that the active compounds interfered with intermediates Z•/D•, which are localized on the donor side of PSII. Fluorescence spectroscopy suggested that the mechanism of inhibitory action of the prepared compounds possibly involves interactions with aromatic amino acids present in photosynthetic proteins.
Acknowledgments
This work was supported by the Slovak Grant Agency VEGA 1/0612/11 and VEGA 1/0041/15, the Project APVV-0061-11 and The Austrian Science Fund (FWF) grants P23609 and P21437.
Author Contributions
F.Š., F.G., P.M., D.D., K.K., M.Z. and J.D. performed experiments. F.Š., F.G., K.K. and J.G. analysed results and wrote the manuscript. All authors contributed to the paper and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Khan, S.A.; Siddiqui, N.; Verma, S.P.; Mullick, P.; Alam, O.J. Synthesis and in vitro antimicrobial activity of novel N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine arboxamide derivatives of benzothiazole class. J. Enzym. Inhib. Med. Chem. 2011, 26, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Cacic, M.; Trkovnik, M.; Cacic, F.; Has-Schon, E. Synthesis and antimicrobial activity of some derivatives of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide. Molecules 2006, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M. Synthesis of a new group of aliphatic hydrazide derivatives and the correlations between their molecular structure and biological activity. Molecules 2012, 17, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Gangwal, R.; Sangamwar, A.T.; Jain, R. Synthesis, biological evaluation and 3D-QSAR study of hydrazide, semicarbazide and thiosemicarbazide derivatives of 4-(adamantan-1-yl)quinoline as anti-tuberculosis agents. Eur. J. Med. Chem. 2014, 85, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; de Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of N(2)-acyl isonicotinic acid hydrazide derivatives. Med. Chem. 2013, 9, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Refat, H.M.; Fadda, A.A. Synthesis and antimicrobial activity of some novel hydrazide, benzochromenone, dihydropyridine, pyrrole, thiazole and thiophene derivatives. Eur. J. Med. Chem. 2013, 70, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Hana, H.Y. Synthesis of progesterone heterocyclic derivatives of potential antimicrobial activity. Acta Pharm. 2008, 58, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Su, H.; Jiang, J.; Yang, X.; Nishida, Y. Design, synthesis and bioactivity of N-glycosyl-N′(5-substituted phenyl-2-furoyl) hydrazide derivatives. Int. J. Mol. Sci. 2014, 15, 6741–6756. [Google Scholar] [CrossRef] [PubMed]
- Ozçelik, A.B.; Gökçe, M.; Orhan, I.; Kaynak, F.; Sahin, M.F. Synthesis and antimicrobial, acetylcholinesterase and butyrylcholinesterase inhibitory activities of novel ester and hydrazide derivatives of 3(2H)-pyridazinone. Arzneimittelforschung 2010, 60, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Bartzatt, R.; Cirillo, S.L.; Cirillo, J.D. Small molecule hydrazide agents to inhibit growth and proliferation of mycobacterium tuberculosis. Med. Chem. 2012, 8, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Modzelewska-Banachiewicz, B.; Wiese, M.; Eljaszewicz, A.; Michałkiewicz, J. Synthesis and anti-inflammatory activity of hydrazide derivatives of 2-methylidene-1,4-dicarboxybutanoic acid. Acta Pol. Pharm. 2012, 69, 1390–1394. [Google Scholar] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Narasimhan, B. Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium. Mini Rev. Med. Chem. 2013, 13, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; de Flora, S.; Cavanna, M.; Pino, A.; Robbiano, L.; Bennicelli, C.; Brambilla, G. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res. 1981, 41, 1469–1482. [Google Scholar] [PubMed]
- Kak, S.N.; Kaul, B.L. Mutagenic activity of hydrazine and its combinations with maleic hydrazide and X-rays in barley. Cytobios 1975, 12, 123–128. [Google Scholar] [PubMed]
- Levi, B.Z.; Kuhn, J.C.; Ulitzur, S. Determination of the activity of 16 hydrazine derivatives in the bioluminescence test for genotoxic agents. Mutat. Res. 1986, 173, 233–237. [Google Scholar] [CrossRef]
- Mazza, M.; Montanari, L.; Pavanetto, F. Phytotoxicity of hydrazones of aromatic aldehydes. Farmaco Sci. 1976, 31, 334–344. [Google Scholar] [PubMed]
- Hendrix, J.A.; Hemmerle, H.; Urmann, M.; Shutske, G.M.; Strupczewski, J.T.; Bordeau, K.J.; Jurcak, J.G.; Nieduzak, T.R.; Jackson, S.A.; Angell, P.; et al. Novel Heterocyclic Substituted Carbonyl Derivatives and Their Use as Dopamine D3 Receptor Ligands. US Patent 20090247509 A1; filed 4 November 2004, and issued 6 April 2004,
- Saleh, M.A. Mutagenic and carcinogenic effects of pesticides. J. Environ. Sci. Health B 1980, 15, 907–927. [Google Scholar] [CrossRef] [PubMed]
- Swietlinska, Z.; Zuk, J. Cytotoxic effects of maleic hydrazide. Mutat. Res. 1978, 55, 15–30. [Google Scholar] [CrossRef]
- Heath, R.L. Hydrazine as an electron donor to the water-oxidation site in photosynthesis. Biochim. Biophys. Acta 1971, 245, 160–164. [Google Scholar] [CrossRef]
- Messinger, J.; Renger, G. Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox states S0, S−1, and S−2 of the water oxidase in isolated spinach thylakoids. Biochemistry 1993, 32, 9379–9386. [Google Scholar] [CrossRef] [PubMed]
- Förster, V.; Junge, W. On the action of hydroxylamine, hydrazine and their derivatives on the water-oxidizing complex. Photosynth. Res. 1986, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Messinger, J.; Renger, G. The reactivity of hydrazine with photosystem II strongly depends on the redox state of the water oxidizing system. FEBS Lett. 1990, 277, 141–146. [Google Scholar] [CrossRef]
- He, P. The differences between hydroxylamine and hydrazine in affecting oxygen evolution of photosynthetic water splitting system in tobacco. Acta Phytophysiol. Sin. 1996, 22, 165–170. [Google Scholar]
- Koske, T.J.; Svec, L.V. Some effects of maleic hyrazide on light reactions of photosynthesis in isolated chloroplasts from Phaseolus vulgaris plants. Can. J. Plant Sci. 1975, 55, 145–149. [Google Scholar] [CrossRef]
- Haveman, J.; Duysens, L.N.M.; van Der Geest, T.C.M.; van Gorkom, H.J. Hydrazobenzene oxidation by 2,6-dichlorophenol-indophenol in a photoreaction catalyzed by system I of photosynthesis. Hydrazine compounds as donors for photosystem II. Biochim. Biophys. Acta 1972, 283, 316–327. [Google Scholar] [CrossRef]
- Gasparova, R.; Zbojek, D.; Lacova, M.; Kral’ova, K.; Gatial, A.; Horvath, B.; Krutosikova, A. Reactions of substituted furo [3,2-b]pyrrole-5-carboxhydrazides and their biological activity. Cent. Eur. J. Chem. 2005, 3, 622–646. [Google Scholar]
- Meyer, S.T.; Webster, J.D.; Young, D.H. Synergistic Algicidal Compositions Including Hydrazone Derivatives and Copper. US Patent 08906829 B2; filed 18 August 2011, and issued 9 December 2014,
- Heytler, P.G.; Prichard, W.W. A new class of uncoupling agents-carbonyl cyanide phenylhydrazones. Biochem. Biophys. Res. Commun. 1962, 7, 272–275. [Google Scholar] [CrossRef]
- Benz, R.; McLaughlin, S.S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys. J. 1983, 41, 381–398. [Google Scholar] [CrossRef]
- Avila, C.M.; Lopes, A.B.; Gonçalves, A.S.; da Silva, L.L.; Romeiro, N.C.; Miranda, A.L.; Sant’Anna, C.M.; Barreiro, E.J.; Fraga, C.A. Structure-based design and biological profile of (E)-N-(4-Nitrobenzylidene)-2-naphthohydrazide, a novel small molecule inhibitor of IκB kinase-β. Eur. J. Med. Chem. 2011, 46, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.M.; Pereira, S.L.; Kümmerle, A.E.; Leal, D.M.; Tesch, R.; de Sant’Anna, C.M.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.R.; Ram, K.R.; Rao, R.V.; Yadav, J.S.; Rao, J.V.; Velatooru, L.R. Synthesis of new S-alkylated-3-mercapto-1,2,4-triazole derivatives bearing cyclic amine moiety as potent anticancer agents. Lett. Drug Des. Discov. 2012, 9, 276–281. [Google Scholar] [CrossRef]
- Grimster, N.P.; Connelly, S.; Baranczak, A.; Dong, J.; Krasnova, L. Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5658. [Google Scholar] [CrossRef] [PubMed]
- Schwenker, G. The reaction of isonicotinic acid hydrazide with 2,4-dinitrochlorobenzene. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1958, 291, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Defusco, A.A.; Strauss, M.J. Displacement-cyclization reactions of mono-substituted hydrazines with chloronitrobenzenes and chloronitropyrimidines. New routes to 8-azapurine and benzopyrazole derivatives. J. Heterocycl. Chem. 1981, 18, 351–355. [Google Scholar] [CrossRef]
- Karrer, F.; Hall, R.G. Cyanohydrazone Derivatives. US Patent 6417386 B2; filed 13 February 2002, and issued 19 July 2002,
- Hemming, K.; Luheshi, A.B.N.; Redhouse, A.D.; Smaalley, R.K.; Thompson, J.R.; Kennewell, P.D.; Westwood, R. 1,3-Dipolar cycloadditions of 2-Ethoxy- and 2-(ethylthio)-1-azetines with nitrile oxides, nitrile ylides and nitrilimines: An unexpected 1,2,4-triazole formation. Tetrahedron 1993, 49, 4383–4408. [Google Scholar] [CrossRef]
- Huisgen, I.; Grashey, R.; Aufderhaar, E.; Kunz, R.; Siedel, M. 1.3-Dipolare Cycloadditionen, VI. Anlagerung der Nitrilimine an Azomethine und Isocyanate. Chem. Ber. 1964, 97, 1085–1095. [Google Scholar] [CrossRef]
- Polumbrik, O.M.; Ryabokon, I.G.; Matkovskii, L.N. Perfluorophenyl-containing verdazyl radicals. Chem. Heterocycl. Comp. 1980, 16, 882–885. [Google Scholar] [CrossRef]
- Doležal, M.; Miletín, M.; Kuneš, J.; Kráľová, K. Substituted Amides of Pyrazine-2-carboxylic acids: Synthesis and Biological Activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-aubstituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Bobal, P.; Zadrazilova, I.; Govender, R.; Pesko, M.; Keltosova, S.; Koleckarova, P.; Marsalek, P.; Imramovsky, A.; Coffey, A.; et al. Antimycobacterial and photosynthetic electron transport inhibiting activity of ring-substituted 4-arylamino-7-chloroquinolinium chlorides. Molecules 2013, 18, 10648–10670. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, P.; Keltosova, K.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, O.; Dolezal, M.; Paterova, P.; Kubicek, V.; Pesko, M.; Kunes, J.; Coffey, A.; Guo, J.; Kralova, K. N-Substituted 5-amino-6-methylpyrazine-2,3-dicarbonitriles: Microwave-assisted synthesis and biological properties. Molecules 2014, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Fedtke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: Berlin-Heidelberg, Germany; New York, NY, USA, 1982. [Google Scholar]
- Govindjee, E. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Debus, R.J.; Barry, B.A.; Babcock, G.T.; McIntosh, L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc. Natl. Acad. Sci. USA 1988, 85, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Debus, R.J.; Barry, B.A.; Sithole, I.; Babcock, G.T.; McIntosh, L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry 1988, 27, 9071–9074. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.J. Application of ESR in photosynthesis. Phys. Rep. 1979, 54, 75–200. [Google Scholar] [CrossRef]
- Pavlíková, S.; Šeršeň, F.; Jesenák, K.; Gaplovska, K.; Čík, G. Efficacy of modified natural zeolites in the protection against the damaging effect of 4-chlorophenol on algal growth. Fresenius Environ. Bull. 2010, 19, 3055–3058. [Google Scholar]
- Šeršeň, F.; Balgavý, P.; Devínsky, F. Electron spin resonance study of chloroplast photosynthetic activity in the presence of amphiphilic amines. Gen. Physiol. Biophys. 1990, 9, 625–633. [Google Scholar] [PubMed]
- Šeršeň, F.; Kráľová, K.; Macho, V. New findings about the inhibitory action of phenylcarbamates and phenylthiocarbamates on photosynthetic apparatus. Pestic. Biochem. Physiol. 2000, 68, 113–118. [Google Scholar] [CrossRef]
- Šeršeň, F.; Kráľová, K.; Peško, M.; Cigáň, M. Effect of Pb2+ ions on photosynthetic apparatus. Gen. Physiol. Biophys. 2014, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).