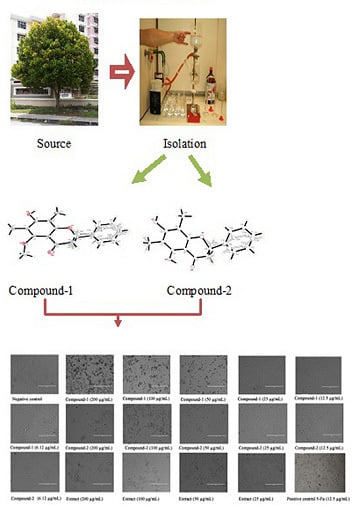
Isolation, Characterization, Crystal Structure Elucidation of Two Flavanones and Simultaneous RP-HPLC Determination of Five Major Compounds from Syzygium campanulatum Korth
Abstract
:1. Introduction
2. Results and Discussion







| Parameters | Crystal Data | |
|---|---|---|
| Compound 1 | Compound 2 | |
| Formula | C18H18O4 | C17H16O4 |
| Formula Weight | 298.32 | 284.30 |
| Crystal System | Monoclinic | Monoclinic |
| Space group | P21/c (No. 14) | P21/c (No. 14) |
| Alpha (°) | 90 | 90 |
| Beta (°) | 105.966 (3) | 100.1616 (17) |
| Gamma (°) | 90 | 90 |
| Unit cell dimensions a (A°) | 12.7683 (14) | 4.8133 (1) |
| b (A°) | 17.2730 (15) | 24.5685 (6) |
| c (A°) | 7.2728 (7) | 12.7303 (4) |
| V (A°3) | 1542.1 (3) | 1481.82 (7) |
| Z | 4 | 4 |
| Density (calcd) (gm/cm3) | 1.285 | 1.274 |
| Mu(CuKa) [/mm] | 0.090 | 0.744 |
| F(000) | 632 | 600 |
| Crystal Size (mm) | 0.479 × 0.167 × 0.122 | 0.03 × 0.09 × 0.32 |
| Temperature (K) | 294 | 294 |
| Radiation (A°) | MoKa | CuKa 1.54178 |
| θ Min, max (°) | 1.659, 27.499 | 4.0, 70.2 |
| Dataset | −16:16; −22:22; −8: 9 | −5:5; −28:29; −15:14 |
| Tot., Uniq. Data | 15,650, 3514 | 26,315, 2751 |
| R (int) | 0.0416 | 0.037 |
| Observed data [I > 2.0 sigma(I)] | 2558 | 2104 |
| Nref, Npar | 3514, 240 | 2751, 240 |
| R, wR2, S | 0.0775, 0.2521, 0.975 | 0.056, 0.1714, 1.10 |
| Compound-1 | Compound-2 | ||
|---|---|---|---|
| Atoms | Bond Angles (°) | Atoms | Bond Angles (°) |
| C1-O1-C9 | 115.76(16) | C9-O1-C1-C2 | 154.0(3) |
| C2-C3-C4 | 123.12(16) | C1-O1-C9-C8 | 52.9(4) |
| C3-C4-C17 | 120.72(17) | C1-O1-C9-C10 | 172.7(3) |
| C4-C5-C6 | 122.19(16) | O1-C1-C2-C16 | 1.5(3) |
| C5-C4-C3 | 117.90(16) | C6-C1-C2-C3 | 2.1(3) |
| C1-C6-C5 | 116.81(15) | C1-C2-C3-O2 | 179.14(18) |
| C3-C2-C16 | 122.27(15) | C16-C2-C3-C4 | 178.51(19) |
| O2-C3-C2 | 121.25(16) | O2-C3-C4-C5 | 178.59(18) |
| C3-C4-C17 | 120.72(17) | C2-C3-C4-C17 | 179.1(2) |
| C3-C4-C5 | 117.90(16) | C3-C4-C5-C6 | 2.6(3) |
| C17-C4-C5 | 121.38(17) | C17-C4-C5-O3 | 1.8(3) |
| O3-C5-C6 | 120.01(15) | O3-C5-C6-C1 | 178.83(18) |
| O2--H1, O2--C3 | 0.88(3), 1.359(2) | O2--H1, O2--O4 | 0.87(3),1.91(4) |
| O3--C5, O3--C18 | 1.376(2), 1.425(3) | O3--H1, O3--O4 | 0.92(3),1.73(3) |
| C16--H16A | 0.9600 | C16--H16A--O1 | 0.9600, 2.3400 |
| C17--H17A | 0.9600 | C17--H17A--O3 | 0.9600, 2.3500 |

| Plant | Compound 1 (%) | Compound 2 (%) | Compound 3 (%) | Compound 4 (%) | Compound 5 (%) | Total (%) |
|---|---|---|---|---|---|---|
| A | 0.12 | 0.34 | 0.09 | 49.33 | 5.27 | 55.16 |
| B | 1.39 | 0.34 | 5.03 | 60.49 | 5.70 | 72.97 |
| C | 2.99 | 0.52 | 8.97 | 34.54 | 2.85 | 49.89 |
| D | 2.04 | 1.00 | 9.37 | 7.355 | 2.46 | 22.24 |
| E | 0.30 | 0.20 | 0.19 | 52.23 | 4.01 | 56.94 |
| Parameters | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Retention time (min) | 5.1 | 7.3 | 10.0 | 15.1 | 16.0 |
| Linearity range (µg·mL−1) | 0.78–200 | 0.78–200 | 0.78–200 | 12.5–200 | 3.1–200 |
| Slope | 47.90 | 54.57 | 54.66 | 1.208 | 5.182 |
| Intercept | 31.89 | −73.75 | −81.22 | −1.25 | −11.60 |
| Correlation coefficient | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| LOD (µg·mL−1) | 0.13 | 0.03 | 0.05 | 0.73 | 0.38 |
| LOQ (µg·mL−1) | 0.40 | 0.10 | 0.14 | 2.23 | 1.17 |
| Accuracy (%) | 95–92 | 99–91 | 101–98 | 96–92 | 94–92 |


3. Experimental Section
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Cell Culture and Cell Lines
3.4. Extraction
3.5. Isolation of Compounds (1–4)
3.6. Crystallization
3.7. Characterization of Compounds
3.7.1. UV-Visible Spectroscopy
3.7.2. FTIR Spectroscopy
3.7.3. NMR Spectroscopy
3.7.4. X-ray Crystallography
3.7.5. LC-EIMS Analysis
3.7.6. Preparation of Stock Solutions for HPLC Analysis
3.8. HPLC Method Validation
3.8.1. Selection of Detection Wavelength
3.8.2. Linearity
3.8.3. Accuracy
3.8.4. Precision
3.8.5. Robustness
3.8.6. Specificity
3.8.7. Reproducibility
3.9. Quantification of Compounds (1–5) in Mixture and Extracts
3.10. Cells Proliferation Assay Using MTT
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tuiwawa, S.H.; Craven, L.A.; Sam, C.; Crisp, M.D. The genus Syzygium (Myrtaceae) in Vanuatu. Blumea 2013, 58, 53–67. [Google Scholar] [CrossRef]
- Chang, C.W.; Wu, T.S.; Hsieh, Y.S.; Kuo, S.C.; Chao, P.D. Terpenoids of Syzygium formosanum. J. Nat. Prod. 1999, 62, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Abdalrahim, F.A.A.; Khalid, M.A.; Salman, A.A.; Mohammad, J.S.; Zhari, I.; Amin, M.S.A.M. Syzygium aromaticum extracts as good source of betulinic acid and potential anti-breast cancer. Braz. J. Pharmacog. 2012, 22, 335–343. [Google Scholar]
- Miyazawa, M.; Hisama, M. Antimutagenic activity of phenylpropanoids from clove (Syzygium aromaticum). J. Agric. Food Chem. 2003, 51, 6413–6422. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.H.; Ismail, Z.; Aisha, A.F.A.; Al-Suede, F.S.R.; Hamil, M.S.R.; Hashim, S.; Saeed, M.A.A.; Laghari, M.; Abdul Majid, A.M.S. Isolation, Characterization, Crystal Structure Elucidation, and Anticancer Study of Dimethyl Cardamonin, Isolated from Syzygium campanulatum Korth. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.N.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Kamel, M.S. One new flavonoid xyloside and one new natural triterpene rhamnoside from the leaves of Syzygium grande. Phytochem. Lett. 2014, 10, 86–90. [Google Scholar] [CrossRef]
- Mir, Q.Y.; Ali, M.; Alam, P. Lignan derivatives from the stem bark of Syzygium cumini (L.) Skeels. Nat. Prod. Res. 2009, 23, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Mi, Y.; Chen, W.; Liu, Q.; Wang, J.; Lou, L.; Zhao, W. Alkyl phloroglucinol derivatives from Syzygium levinei and their differentiation-inducing activity. Planta Med. 2006, 72, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Orii, Y.; Nonaka, G.I.; Nishioka, I.; Kouno, I. Syzyginin-A and Syzyginin-B, 2 Ellagitannins from Syzygium-aromaticum. Phytochemistry 1996, 43, 1345–1348. [Google Scholar] [CrossRef]
- Qing-Hua, H.; Dong-Mei, W.; Zhong-Bin, C.; Xue, Y.; Xin-Jun, X.; Jun, W.; Sheng, Y. Chemical constituents from the leaves and twigs of Syzygium tetragonum Wall. Biochem. Syst. Ecol. 2012, 41, 3–5. [Google Scholar]
- Wilma, P.R.; Leonardo, L.B.; Nilda, M.A.; Pedro, H.F.; José, R.P. Chemical variability in the essential oils from leaves of Syzygium Jambos. Braz. J. Pharmacog. 2013, 22, 433–440. [Google Scholar]
- Stanely, M.P.; Menon, V.P.; Pari, L. Hypoglycaemic activity of Syzigium cumini seeds: Effect on lipid peroxidation in alloxan diabetic rats. J. Ethnopharmacol. 1998, 61, 1–7. [Google Scholar]
- Muruganandan, S.; Srivastava, K.; Chandra, S.; Tandan, S.K.; Lal, J.; Raviprakash, V. Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia 2001, 72, 369–375. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, E.H.; Hong, S.H.; Song, H.J.; Shin, M.K.; Kim, S.H.; Shin, T.Y. Effect of Syzygium aromaticum extract on immediate hypersensitivity in rats. J. Ethnopharmacol. 1998, 60, 125–131. [Google Scholar] [CrossRef]
- De Lima, T.C.M.; Klüeger, P.A.; Pereira, P.A.; Macedo-Neto, W.P.; Morato, G.S.; Farias, M.R. Behavioral effects of crude and semi-purified extracts of Syzygium cuminii linn. skeels. Phytother. Res. 1998, 12, 488–493. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [PubMed]
- Djipa, C.D.; Delmée, M.; Quetin-Leclercq, J. Antimicrobial activity of bark extracts of Syzygium Jambos (L.) Alston (Myrtaceae). J. Ethnopharmacol. 2000, 71, 307–313. [Google Scholar] [CrossRef]
- Abdalrahim, F.A.A.; Zhari, I.; Khalid, M.A.; Jamshed, M.S.; Gheniya, G.; Amin, M.S.A.M. Syzygium campanulatum korth methanolic extract inhibits angiogenesis and tumor growth in nude mice. BMC. Complement. Altern. Med. 2013, 13, 168–179. [Google Scholar]
- Waller, G.R.; Nowacki, E.K. Alkaloid Biology and Metabolism in Plants; Plenum Press: New York, NY, USA, 1978. [Google Scholar]
- Starlin, T.; Arul, R.C.; Ragavendran, P.; Gopalakrishnan, V.K. Phytochemical screening, Functional Groups and Element Analysis of Tylophora Pauciflora Wight and Arn. Int. Res. J. Pharm. 2012, 3, 180–183. [Google Scholar]
- Iqbal, M.A.; Haque, R.A.; Ahamed, M.B.K.; Abdul, M.A.M.S.; Al-Rawi, S. Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag(I) N-heterocyclic carbene complexes. Med. Chem. Res. 2013, 22, 2455–2466. [Google Scholar] [CrossRef]
- Terry, M.I.; James, C.R.; Christian, C.M.; Mathew, J.S.; Mark, D.B.; Robert, J.O.T. Instrumental Data for Drug Analysis; CRC Press, Taylor & Frances Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006; Volume 1, p. 4688. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Shashank, K.; Abhay, K.P. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Nicolas, F.; Isabelle, R.; Edmond, D.H.; Joe¨lle, Q.L. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 701–715. [Google Scholar]
- Bruno, D.M.; Ana, M.; Ramon, J.E. 7-Hydroxy-5-methoxy-6,8-dimethylflavanone: A natural flavonoid. Acta Crystallogr. 2008, C64, 353–356. [Google Scholar]
- Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Halogenated flavanones as potential apoptosis-inducing agents: Synthesis and biological activity evaluation. Eur. J. Med. Chem. 2012, 58, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Xiaoying, W.; Ningbo, G.; Shiying, Y.; Guanhua, D.; Yang, L. Studies on Solvatomorphism of Betulinic Acid. J. Pharm. Sci. 2014, 103, 2696–2703. [Google Scholar]
- ICH Q2 (R1). Validation of Analytical Procedures:Text and Methodology. In Proceedings of International Conference on Harmonization, Geneva, Switzerland; 2005.
- Nursyazura, K.; Abdalrahim, F.A.A.; Zhari, I. Reverse Phase High Performance Liquid Chromatography for the Quantification of Eurycomanone in Eurycoma longifolia Jack (Simaroubaceae) Extracts and their Commercial Products. Trop. J. Pharm. Res. 2014, 13, 801–807. [Google Scholar]
- Al-Suede, F.S.R.; Elham, F.; Mohamed, B.; Ismail, Z.; Majid, A.S.A.; Abdul Majid, A.M.S. Marked antitumor activity of cat’s whiskers tea (Orthosiphon stamineus) extract in orthotopic model of human colon tumor in nude mice. J. Biochem. Technol. 2012, 3, 170–176. [Google Scholar]
- Sample Availability: Samples of the compounds (2S)-7-Hydroxy-5-methoxy-6,8-dimethyl flavanone, (S)-5,7-dihydroxy-6,8-dimethyl flavanone, (E)-2ʹ,4ʹ-dihydroxy-6ʹ-methoxy-3ʹ,5ʹ-dimethylchalcone, and Betulinic acid are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, A.H.; Ismail, Z.; Al-Suede, F.S.R.; Aisha, A.F.A.; Hamil, M.S.R.; Saeed, M.A.A.; Laghari, M.; Majid, A.M.S.A. Isolation, Characterization, Crystal Structure Elucidation of Two Flavanones and Simultaneous RP-HPLC Determination of Five Major Compounds from Syzygium campanulatum Korth. Molecules 2015, 20, 14212-14233. https://doi.org/10.3390/molecules200814212
Memon AH, Ismail Z, Al-Suede FSR, Aisha AFA, Hamil MSR, Saeed MAA, Laghari M, Majid AMSA. Isolation, Characterization, Crystal Structure Elucidation of Two Flavanones and Simultaneous RP-HPLC Determination of Five Major Compounds from Syzygium campanulatum Korth. Molecules. 2015; 20(8):14212-14233. https://doi.org/10.3390/molecules200814212
Chicago/Turabian StyleMemon, Abdul Hakeem, Zhari Ismail, Fouad Saleih Resq Al-Suede, Abdalrahim F. A. Aisha, Mohammad Shahrul Ridzuan Hamil, Mohammed Ali Ahmed Saeed, Madeeha Laghari, and Amin Malik Shah Abdul Majid. 2015. "Isolation, Characterization, Crystal Structure Elucidation of Two Flavanones and Simultaneous RP-HPLC Determination of Five Major Compounds from Syzygium campanulatum Korth" Molecules 20, no. 8: 14212-14233. https://doi.org/10.3390/molecules200814212
APA StyleMemon, A. H., Ismail, Z., Al-Suede, F. S. R., Aisha, A. F. A., Hamil, M. S. R., Saeed, M. A. A., Laghari, M., & Majid, A. M. S. A. (2015). Isolation, Characterization, Crystal Structure Elucidation of Two Flavanones and Simultaneous RP-HPLC Determination of Five Major Compounds from Syzygium campanulatum Korth. Molecules, 20(8), 14212-14233. https://doi.org/10.3390/molecules200814212