Antigenotoxicity and Tumor Growing Inhibition by Leafy Brassica carinata and Sinigrin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Leaf Glucosinolate Content Determination

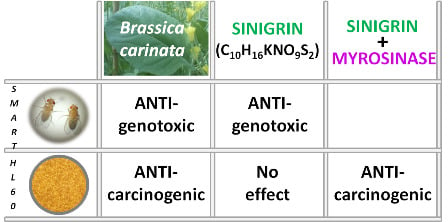
2.2. In Vivo Assays
2.2.1. Toxicity Assays
| Toxicity | |||||||
|---|---|---|---|---|---|---|---|
| Survival a % Treatments | |||||||
| Controls | Simple | B. carinata (mg·mL−1) | Simple | Combined b | Sinigrin (mM c) | Simple | Combined b |
| H2O | 100 | 1.25 | 55.33 * | 37.78 * | 0.60 | 56.44 * | 25.56 * |
| H2O2 (0.12 M) | 37.78 * | 2.50 | 53.33 * | 30 * | 1.20 | 78.22 * | 19.33 * |
| 5.00 | 47.56 * | 36.89 * | 2.40 | 61.56 * | 47.33 * | ||
| 4.81 | 76.89 * | 60.22 * | |||||
| Average | 52.07 | 34.89 | 68.27 | 38.11 | |||
2.2.2. Genotoxicity Assays
| Genotoxicity | |||||
|---|---|---|---|---|---|
| Mutation Rate (Spots per Wing) Diagnosis a | |||||
| Treatment | N° of wings | Small single spots 1–2 cells; m = 2 | Large single spots > 2 cells; m = 5 | Twin spots m = 5 | Total spots m = 2 |
| Controls | |||||
| H2O | 212 | 0.26 (54) | 0.04 (8) | 0.03 (5) | 0.32 (67) |
| H2O2 (0.12 M) | 168 | 0.60 (94) + | 0.07 (11) − | 0.06 (4) − | 0.65 (109) + |
| Plant Material: Brassica carinata (mg·mL−1) | |||||
| 1.25 | 40 | 0.01 (4) − | 0.03 (1) − | 0.03 (1) − | 0.15 (6) − |
| 2.50 | 48 | 0.15 (7) − | 0.04 (2) − | 0.02 (1) − | 0.21 (10) − |
| 5.00 | 48 | 0.13 (6) − | 0.02 (1) − | 0.02 (1) − | 0.19 (8) − |
| Single Compound: Sinigrin (mM) b | |||||
| 0.60 | 40 | 0.18 (7) − | 0.03 (1) − | 0 | 0.20 (8) − |
| 1.20 | 34 | 0.12 (4) − | 0.03 (1) − | 0.03 (1) − | 0.18 (6) − |
| 2.40 | 36 | 0.08 (3) − | 0.03 (1) − | 0 | 0.11 (4) − |
| 4.81 | 28 | 0.14 (4) − | 0 | 0 | 0.14 (4) − |
2.2.3. Antigenotoxicity Assays
| Antigenotoxicity | |||||
|---|---|---|---|---|---|
| Mutation Rate (Spots per Wing) Diagnosis a | |||||
| Treatment | N° of wings | Small single spots 1–2 cells; m = 2 | Large single spots > 2 cells; m = 5 | Twin spots m = 5 | Total spots m = 2 |
| Controls | |||||
| H2O | 212 | 0.26 (54) | 0.04 (8) | 0.03 (5) | 0.32 (67) |
| H2O2 (0.12 M) | 168 | 0.60 (94) + | 0.07 (11) − | 0.06 (4) − | 0.65 (109) + |
| Plant Material: Brassica carinata (mg·mL−1) | |||||
| 1.25 | 40 | 0.11 (4) − | 0.03 (1) − | 0 | 0.14 (5) − |
| 2.50 | 48 | 0.25 (7) − | 0.04 (1) − | 0 | 0.29 (8) − |
| 5.00 | 48 | 0.27 (8) − | 0.03 (1) | 0 | 0.30 (9) − |
| Single Compound: Sinigrin (mM) b | |||||
| 0.60 | 40 | 0.19 (6) − | 0 | 0 | 0.19 (6) − |
| 1.20 | 34 | 0.20 (4) − | 0 | 0 | 0.20 (4) − |
| 2.40 | 36 | 0.25 (1) − | 0 | 0 | 0.25 (1) − |
| 4.81 | 28 | 0.10 (1) − | 0 | 0 | 0.10 (1) − |

2.3. In Vitro Assays

3. Experimental Section
3.1. Plant Material and Chemicals
3.2. HPLC
3.3. Fly Stocks and Crosses
3.4. Toxicity, Genotoxicity and Antigenotoxicity Studies
3.5. Cell Culture and Treatments
Cell Viability Assay
3.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herr, I.; Buchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Jahgangir, M.J.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–34. [Google Scholar] [CrossRef]
- Redovniković, I.R.; Glivetić, T.; Delonga, K.; Vorkapic-Furač, J. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica species for glucosinolate content. J. Environ. Sci. Health Part B 2009, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Appel, K.E.; Lampen, A. Health benefits and possible risks of broccoli—A review. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Zukalová, H.; Vaŝák, J. The role and effect of glucosinolates of Brassica species—A review. Rostl. Vyroba 2002, 48, 175–180. [Google Scholar]
- Schippers, R.R. African Indigenous Vegetables: An Overview of the Cultivated Species; University of Greenwich, Natural Resources Institute: London, UK, 2000. [Google Scholar]
- Tadelle, D.; Alemu, Y.; Moges, H.M.; Fasil, K. Effect of Level of Rapeseed (Brassica carinata) Cake in Rations on Broiler Performance. Livest. Res. Rural Dev. 2003, 15. Available online: http://www.lrrd.org/lrrd15/4/tade154.htm (accessed on 25 June 2015). [Google Scholar]
- Taylor, D.C.; Falk, K.C.; Palmer, C.D.; Hammerlindl, J.; Babic, V.; Mietkiewska, E.; Jadhav, A.; Marillia, E.F.; Francis, T.; Hoffman, T.; et al. Brassica carinata—A new molecular farming platform for delivering bio-industrial oil feedstocks: Case studies of genetic modifications to improve very long-chain fatty acid and oil content in seeds. Biofuels Bioprod. Biorefin. 2010, 4, 538–561. [Google Scholar] [CrossRef]
- De Haro, A.; Domínguez, J.; García, R.; Muñoz, J.; Fernández-Martínez, J.M. Registration of six Ethiopian mustard germplasm lines. Crop Sci. 1998, 38, 558–559. [Google Scholar] [CrossRef]
- Warwick, S.I.; Gugel, R.K.; McDonald, T.; Falk, K.C. Genetic variation of Ethiopian mustard (Brassica carinata A. Braun) germplasm in western Canada. Genet. Resour. Crop Evol. 2006, 53, 297–312. [Google Scholar] [CrossRef]
- Bellostas, N.; Sørensen, J.C.; Sørensen, H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Donkin, S.G.; Eiteman, M.A.; Williams, P.L. Toxicity of glucosinolates and their enzymatic decomposition products to Caenorhabditis elegans. J. Nematol. 1995, 27, 258–262. [Google Scholar] [PubMed]
- Martínez-Ballesta, M.C.; Muries, B.; Moreno, D.A.; Dominguez-Perles, R.; García-Viguera, C.; Carvajal, M. Involvement of a glucosinolate (sinigrin) in the regulation of water transport in Brassica oleracea grown under salt stress. Physiol. Plant 2014, 150, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Lee, H.J. Induction of quinone reductase by allylisothiocyanate (AITC) and the N-acetylcysteine conjugate of AITC in Hepa1c1c7 mouse hepatoma cells. BioFactors 2006, 26, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Allyl isothiocyanates as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994, 54, 1976s–1981s. [Google Scholar] [PubMed]
- Lamy, E.; Schmitz, S.; Krumbein, A.; Mersch-Sundermann, V. Isothiocyanate-containing mustard protects human cells against genotoxins in vitro and in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 726, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Keck, A.S.; Finley, J.W. Cruciferous Vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004, 3, 5–12. [Google Scholar] [CrossRef] [PubMed]
- USDA. Vegetables. Available online: http://www.choosemyplate.gov/food-groups/vegetables.html (accessed on 25 June 2015).
- Agudo, A.; Ibanez, R.; Amiano, P.; Ardanaz, E.; Barricarte, A.; Berenguer, A.; Dolores, C.M.; Dorronsoro, M.; Jakszyn, P.; Larranaga, N.; et al. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur. J. Clin. Nutr. 2008, 62, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Spherix. Generally Recognized as Safe (GRAS) Notification for the Use of Aqueous Broccoli Seed Extract Powder in Food. Available online: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm388588.pdf (accessed on 25 June 2015).
- Sang, J.H.; King, R.C. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J. Exp. Biol. 1961, 38, 793–809. [Google Scholar]
- Miller, R.S.; Thomas, J.L. The effects of larval crowding and body size on the longevity of adult Drosophila melanogaster. Ecology 1958, 39, 118–125. [Google Scholar] [CrossRef]
- Borek, V.; Elberson, L.R.; McCaffrey, J.P.; Morra, M.J. Toxicity of aliphatic and aromatic isothiocyanates to eggs of the black vine weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 1995, 88, 1192–1196. [Google Scholar] [CrossRef]
- Verhoeven, D.T.H.; Verhagen, H.; Goldbohm, R.A.; van den Brant, P.A.; van Poppel, G. A review of mechanisms underlying anticarcinogenicity by Brassica vegetables. Chem. -Biol. Interact. 1997, 103, 79–129. [Google Scholar] [CrossRef]
- Pedroche, J.; Just, M.M.; Lqari, H.; Megias, C.; Giron-Calle, J.; Alaiz, M. Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases. Food Res. Int. 2007, 40, 931–938. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Gicquel, M.; Esnault, M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012, 132, 1003–1009. [Google Scholar] [CrossRef]
- Romero-Jimenez, M.; Campos-Sanchez, J.; Analla, M.; Munoz-Serrano, A.; Alonso-Moraga, A. Genotoxicity and anti-genotoxicity of some traditional medicinal herbs. Mutat. Res. 2005, 585, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, A. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Frei, H.; Würgler, F.E. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. Environ. Mutagen. Relat. Subj. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat. Res. Environ. Mutagen. Relat. Subj. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Baasanjav-Gerber, C.; Hollnagel, H.M.; Brauchmann, J.; Iori, R.; Glatt, H. Detection of genotoxicants in Brassicales using endogenous DNA as a surrogate target and adducts determined by 32P-postlabelling as an experimental end point. Mutagenesis 2011, 26, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Møller, P.; Sørensen, H. Glucosinolates in broccoli stored under controlled atmosphere. J. Am. Soc. Hortic. Sci. 1995, 120, 1069–1074. [Google Scholar]
- Heres-Pulido, M.E.; Duenas-Garcia, I.; Castaneda-Partida, L.; Santos-Cruz, L.F.; Vega-Contreras, V.; Rebollar-Vega, R.; Gomez-Luna, J.C.; Duran-Diaz, A. Genotoxicity studies of organically grown broccoli (Brassica oleracea var. italica) and its interactions with urethane, methyl methanesulfonate and 4-nitroquinoline-1-oxide genotoxicity in the wing spot test of Drosophila melanogaster. Food Chem. Toxicol. 2010, 48, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Musk, S.R.R.; Smith, T.K.; Johnson, I.T. On the cytotoxicity and genotoxicity of allyl and phenethyl isothiocyanates and their parent glucosinolates sinigrin and gluconasturtiin. Mutat. Res. Lett. 1995, 348, 19–23. [Google Scholar] [CrossRef]
- Gonçales, A.L.M.; Lemos, M.; Niero, R.; de Andrade, S.F.; Maistro, E.L. Evaluation of the genotoxic and antigenotoxic potential of Brassica oleracea L. var. acephala DC in different cells of mice. J. Ethnopharmacol. 2012, 143, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; de Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Maruyama, H.; Matsumoto, K.; Ohguchi, K.; Nishizawa, K.; Sakamoto, T.; Araki, Y.; Mishima, S.; Nozawa, Y. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol. Pharm. Bull. 2003, 26, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Jakubikova, J.; Bao, Y.; Sedlak, J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005, 25, 3375–3386. [Google Scholar] [PubMed]
- Ludikhuyze, L.; Rodrigo, L.; Hendrickx, M. The activity of myrosinase from broccoli (Brassica oleracea L. var. italica): Influence of intrinsic and extrinsic factors. J. Food Prot. 2000, 63, 400–403. [Google Scholar] [PubMed]
- Jie, M.; Cheung, W.M.; Yu, V.; Zhou, Y.; Tong, P.H.; Ho, J.W. Anti-proliferative activities of sinigrin on carcinogen-induced hepatotoxicity in rats. PLoS ONE 2014, 9, e110145. [Google Scholar] [CrossRef] [PubMed]
- Øverby, A.; Bævre, M.S.; Thangstad, O.P.; Bones, A.M. Disintegration of microtubules in Arabidopsis thaliana and bladder cancer cells by isothiocyanates. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Mori, Y.; Morishita, Y.; Hara, A.; Ohno, T.; Kojima, T.; Mori, H. Inhibitory effect of sinigrin and indole-3-carbinol on diethylnitrosamine-induced hepatocarcinogenesis in male ACI/N rats. Carcinogenesis 1990, 11, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Lund, E.K.; Parker, M.L.; Clarke, R.; Johnson, I.T. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis 2004, 25, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Lee, H.J. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp. Biol. Med. (Maywood) 2006, 231, 421–430. [Google Scholar] [PubMed]
- Lancashire, P.D.; Bleiholder, H.P.; van den Boom, T.; Langeluddecke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Font, R.M.; del Río-Celestino, M.; Cartea, M.E.; de Haro, A. Quantification of aliphatic and índole glucosinolates in leaves of leaf rape (Brassica napus var. pabularia) by near infrared spectroscopy. Phytochemistry 2005, 66, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wathelet, J.P.; Wagstaffe, P.; Boenke, A. The Certification of the Total Glucosinolate and Sulphur Contents of Three Rapeseeds (Colza), CRMs 190, 366 and 367; European Commission: Brussels, Belgium, 1991. [Google Scholar]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.M.; Yandell, M.D.; Wortman, J.R.; Gabor Miklos, G.L.; Nelson, C.R.; Harihanan, I.K.; Fortini, M.E.; Li, P.W.; Apweiler, R.; Fleischmann, W.; et al. Comparative genomics of the eukaryotes. Science 2000, 287, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Spano, M.A.; Rincon, J.G.; Abraham, S.K.; de Andrade, H.H. The wing somatic mutation and recombination test (SMART) in Drosophila melanogaster: An efficient tool for the detection of genotoxic activity of pure compounds or complex mixtures as well as for studies on antigenotoxicity. Afr. Newslett. Occup. Health Saf. 1996, 6, 9–13. [Google Scholar]
- Lindsley, D.L.; Zimm, G.G. The Genome of Drosophila Melanogaster; Academic Press Inc.: San Diego, CA, USA, 1992. [Google Scholar]
- Zhu, C.Y.; Loft, S. Effect of chemopreventive compounds from Brassica vegetables on NAD(P)H: Quinine reductase and induction of DNA strand breaks in murine hepa1c1c7 cells. Food Chem. Toxicol. 2003, 41, 455–462. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the plant material are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Baena, M.-D.; Tasset, I.; Obregón-Cano, S.; De Haro-Bailon, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Antigenotoxicity and Tumor Growing Inhibition by Leafy Brassica carinata and Sinigrin. Molecules 2015, 20, 15748-15765. https://doi.org/10.3390/molecules200915748
Lozano-Baena M-D, Tasset I, Obregón-Cano S, De Haro-Bailon A, Muñoz-Serrano A, Alonso-Moraga Á. Antigenotoxicity and Tumor Growing Inhibition by Leafy Brassica carinata and Sinigrin. Molecules. 2015; 20(9):15748-15765. https://doi.org/10.3390/molecules200915748
Chicago/Turabian StyleLozano-Baena, María-Dolores, Inmaculada Tasset, Sara Obregón-Cano, Antonio De Haro-Bailon, Andrés Muñoz-Serrano, and Ángeles Alonso-Moraga. 2015. "Antigenotoxicity and Tumor Growing Inhibition by Leafy Brassica carinata and Sinigrin" Molecules 20, no. 9: 15748-15765. https://doi.org/10.3390/molecules200915748