Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Compositions of Tairin and Shima EOs
| No. | Compound | Retention Index | Peak Area (%) | |
|---|---|---|---|---|
| Tairin | Shima | |||
| 1 | m-Cumenol | 917 | 0.16 | 0.15 |
| 2 | α-Thujene | 929 | 4.12 | - |
| 3 | α-Pinene | 934 | 2.02 | - |
| 4 | Norborndadiene | 943 | 0.08 | - |
| 5 | Camphene | 947 | 0.22 | - |
| 6 | Benzaldehyde | 963 | - | 1.59 |
| 7 | Sabinene | 974 | 12.5 | - |
| 8 | β-Pinene | 976 | 3.15 | - |
| 9 | Myrcene | 989 | 0.69 | - |
| 10 | α-Phellandrene | 1002 | 0.31 | - |
| 11 | p-Cymene | 1024 | 13.5 | - |
| 12 | 1,8-Cineole | 1031 | 13.8 | 37.8 |
| 13 | γ-Terpinene | 1059 | 14.5 | - |
| 14 | cis-β-Terpineol | 1068 | 0.55 | - |
| 15 | β-Linalool | 1100 | 0.50 | 17.1 |
| 16 | 2,5-Norbornadiene | 1111 | - | 0.71 |
| 17 | cis-p-Menth-2-en-1-ol | 1121 | 0.59 | - |
| 18 | Borneol | 1154 | 0.31 | 0.11 |
| 19 | Terpinen-4-ol | 1173 | 11.9 | - |
| 20 | γ-Terpineol | 1193 | 1.28 | 3.36 |
| 21 | trans-p-Menth-1-en-3-ol | 1206 | 0.42 | - |
| 22 | Methyl cinnamate | 1233 | 4.24 | 6.32 |
| 23 | Benzylacetone | 1237 | 0.06 | 4.21 |
| 24 | Piperitone | 1248 | 0.03 | 0.1 |
| 25 | Bornyl acetate | 1280 | 0.37 | - |
| 26 | Cumic alcohol | 1287 | 0.18 | - |
| 27 | 2-tert-Butylphenyl pivalate | 1299 | - | 1.23 |
| 28 | Ethyl-3-hydroxy-3-methylbutanote | 1302 | - | 0.09 |
| 29 | Isopiperitenon | 1306 | - | 0.18 |
| 30 | Thymol | 1317 | 0.05 | - |
| 31 | 2-Hydroxy-3,5-dimethylcyclopent-2-en-1-one | 1319 | - | 0.13 |
| 32 | p-Menth-1,4-dien-7-ol | 1324 | 0.07 | - |
| 33 | Cymen-8-ol | 1354 | 0.26 | 0.29 |
| 34 | Carvacrol | 1379 | 1.61 | 1.89 |
| 35 | Caryophyllene | 1411 | 2.40 | - |
| 36 | 2,6-Diethylnitrosobenzene | 1426 | - | 0.69 |
| 37 | α-trans-Bergamoene | 1428 | 0.09 | - |
| 38 | Aristole-9-ene | 1434 | 0.15 | - |
| 39 | α-Humulene | 1446 | 0.38 | - |
| 40 | p-Cymen-7-ol | 1447 | 1.66 | 1.77 |
| 41 | γ-Cadinene | 1505 | 0.42 | - |
| 42 | α-Bulnesene | 1514 | 0.25 | - |
| 43 | Nerolidol | 1559 | 0.38 | - |
| 44 | Caryophyllene oxide | 1680 | 4.96 | 10.4 |
| 45 | α-Zingiberene | 1701 | 0.02 | - |
| Total | 98.18 | 88.12 | ||
2.2. Anti-Oxidant Activities
2.2.1. DPPH and ABTS Radical Scavenging Activities
| Sample | Anti-Oxidant Activities (IC50, μg/mL) | ||||
|---|---|---|---|---|---|
| DPPH | ABTS | Nitric Oxide | Singlet Oxygen | Hydroxyl Radical Scavenging | |
| Tairin | 5.7 ± 0.6 a | 260.5 ± 1.8 b | 66.3 ± 1.6 b | 2145 ± 2.8 c | 69.2 ± 2.1 a |
| Shima | 126.3 ± 1.1 c | 337.1 ± 2.1 c | 704.2 ± 1.4 c | 503 ± 1.7 b | 74.0 ± 1.2 b |
| BHT | 29.1 ± 0.6 b | 45.7 ± 1.7 a | - | - | - |
| Ascorbic acid | - | - | 14.4 ± 0.8 a | - | 72.3 ± 1.2 b |
| Rutin | - | - | - | 54.0 ± 0.2 a | - |
2.2.2. Singlet Oxygen Scavenging (1O2) Activity
2.2.3. Hydroxyl and Nitric Oxide Radical Scavenging Activities
2.3. Anti-Aging Activities
2.3.1. Collagenase and Elastase Assay
2.3.2. Tyrosinase and Hyaluronidase Inhibition Assay

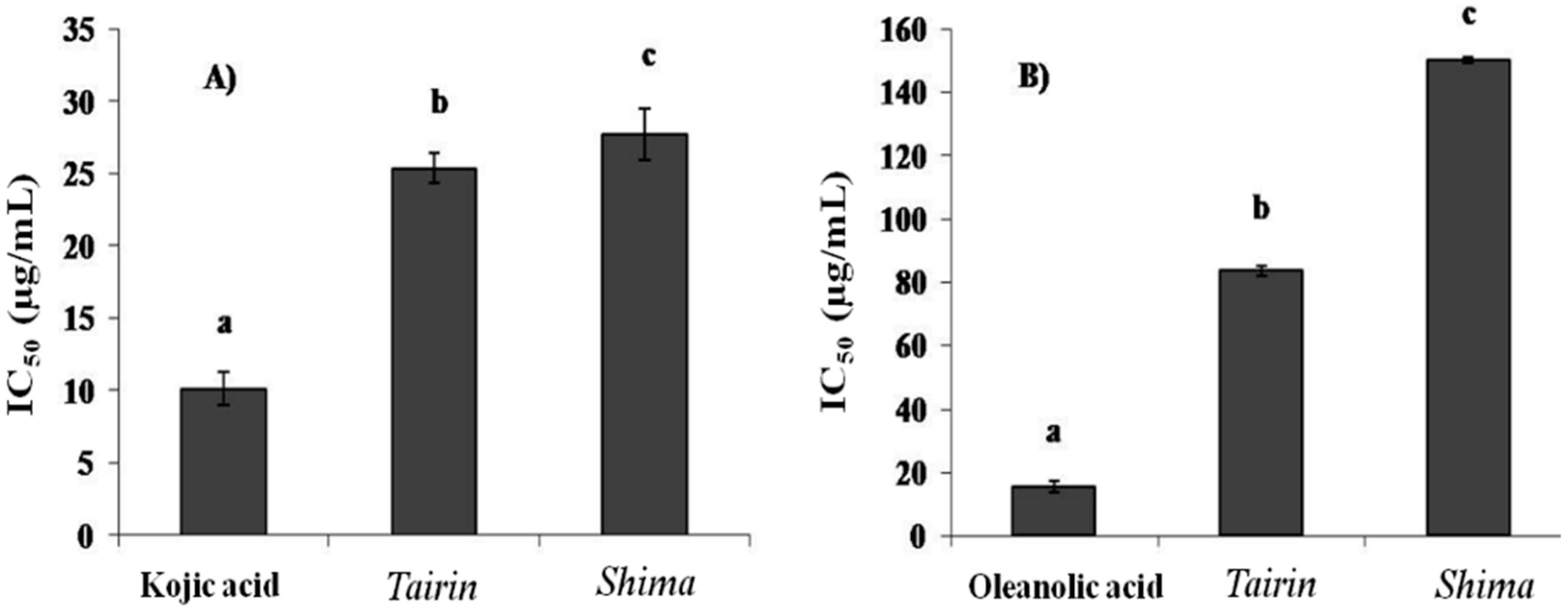
2.3.3. Xanthine Oxidase Inhibition Assay
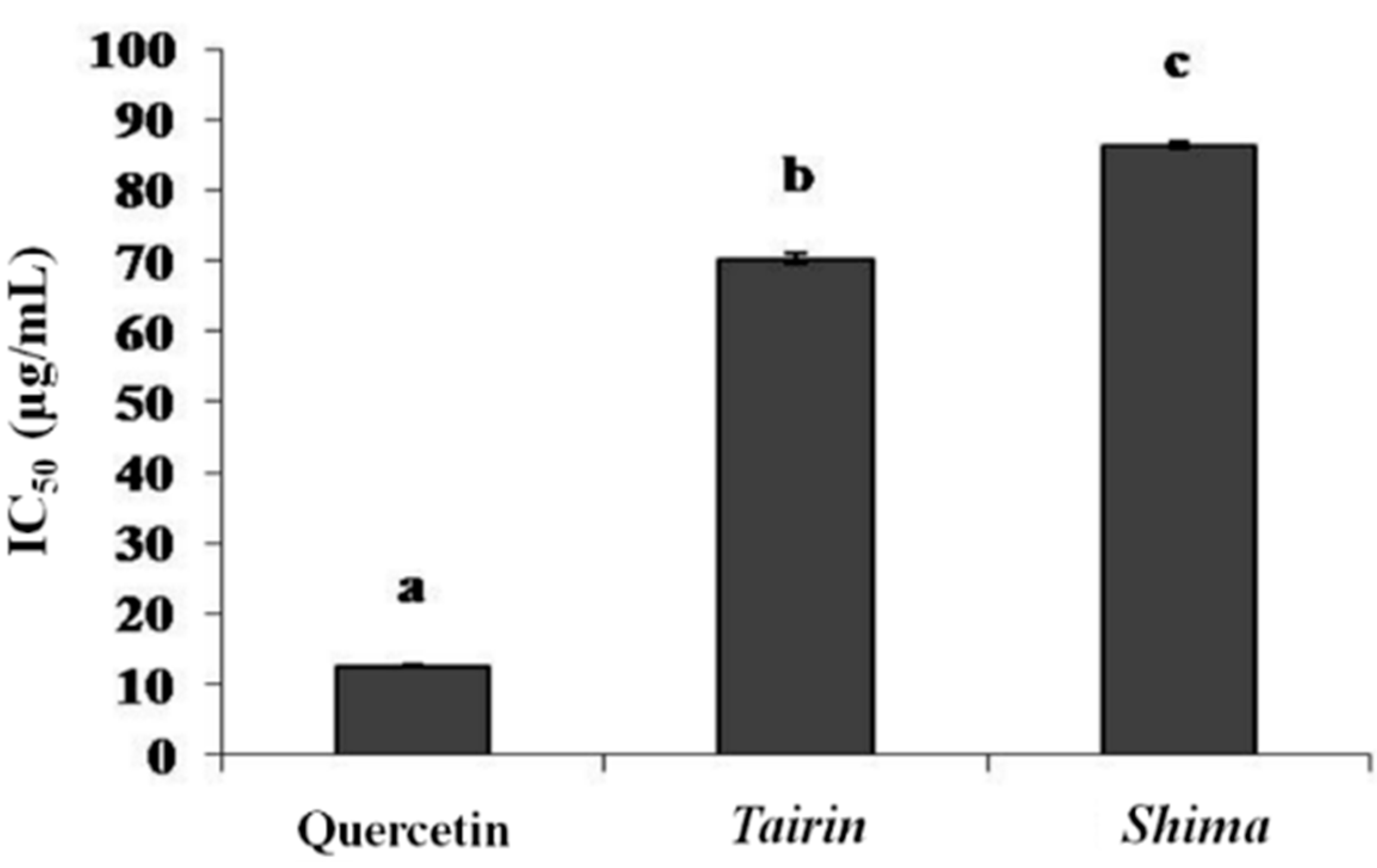
2.4. Anti-Melanogenic Effects of Tairin and Shima EOs
2.4.1. Cell Viability
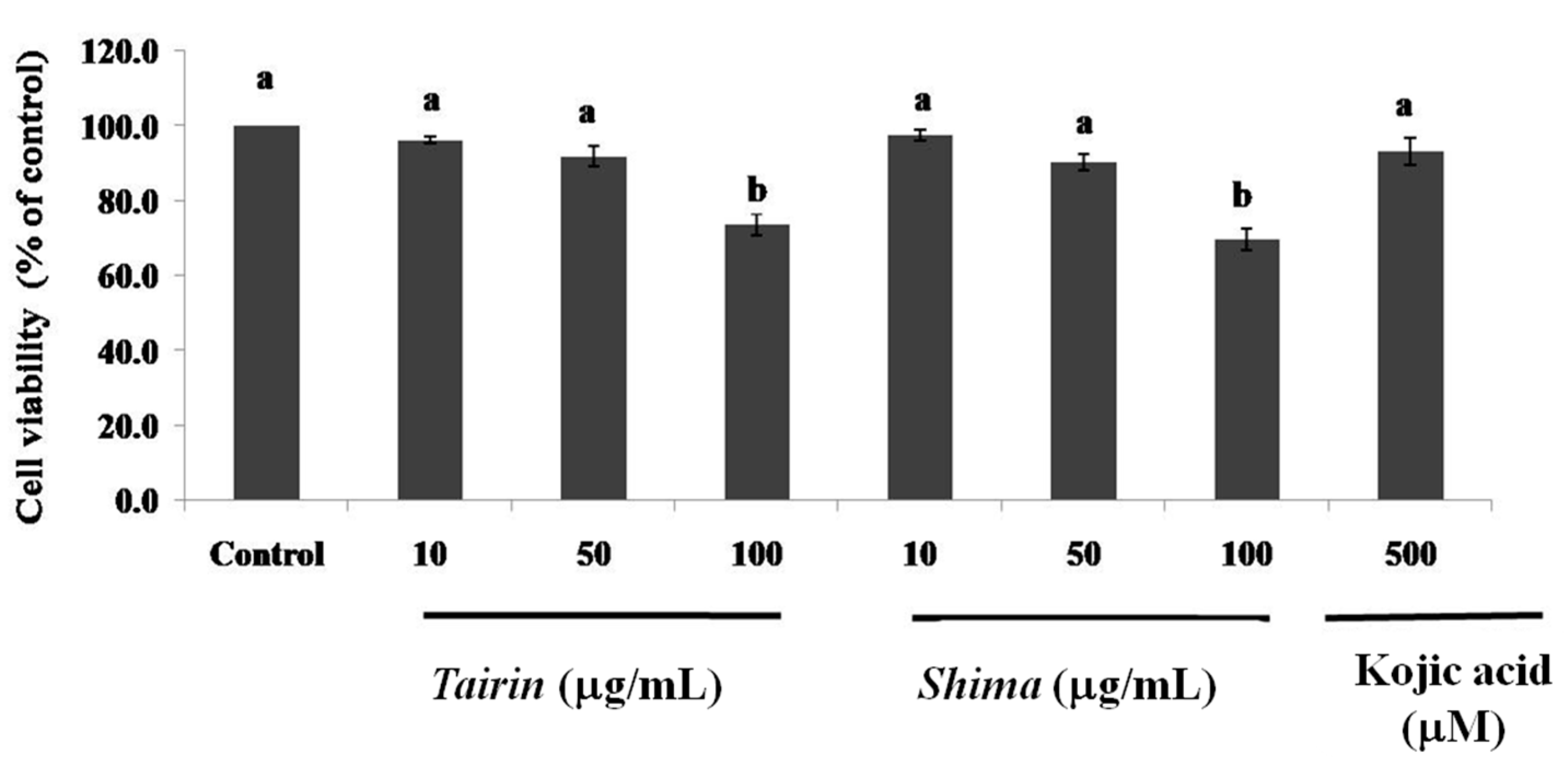
2.4.2. Effects of Tairin and Shima EOs on Melanin Content

2.4.3. Inhibition of the Intracellular Tyrosinase Activity by Tairin and Shima EOs

2.5. Discussion
3. Experimental Section
3.1. General
3.2. Extraction of EO from Alpinia Leaf
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.4. Anti-Oxidant Activity
3.4.1. DPPH Radical Scavenging Assay
3.4.2. ABTS Radical Scavenging Assay
3.4.3. Hydroxyl Radical Scavenging (•OH) Assay
3.4.4. Singlet Oxygen Scavenging (1O2) Assay
3.4.5. Nitric Oxide Scavenging Assay
3.5. Enzymatic Activities
3.5.1. Collagenase Inhibition Assay
3.5.2. Elastase Inhibition Assay
3.5.3. Hyaluronidase Inhibition Assay
3.5.4. Tyrosinase Inhibition Assay
3.5.5. Xanthine Oxidase Inhibition Assay
3.6. Effects of Tairin and Shima EO on Melanin Biosynthesis in B16F10 Melanoma Cells
3.6.1. Cell Culture
3.6.2. Cell Viability
3.6.3. Measurement of Melanin Content
3.6.4. Intracellular Tyrosinase Activity
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, H.E.; Ishihara, A.; Lee, S.G. The effects of Caffeoylserotonin on inhibition of melanogenesis through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16 murine melanoma cells. BMB Rep. 2012, 45, 724–729. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, C.; Garcia-Borron, J.C.; Vanverde, P.; Solano, F.; Lozano, J.A. Tyrosinase isoenzymes in mammalian melanocytes. 1. Biochemical characterization of two melanosomal on tyrosinase from B16 mouse melanoma. Eur. J. Biochem. 1993, 217, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Munene, C.N.; Bansode, R.R.; Bawadi, H.A. Inhibition of matrix metalloproteinase-1 activity by the soybean Bowman-Birk inhibitor. Biotechnol. Lett. 2004, 26, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.; Hashim, P.; Hashim, D.; Halimoon, N.; Muhamad, N.; Majid, N. Anti-elastase, anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Barla, F.; Higashijima, H.; Funai, S.; Sugimoto, K.; Harada, N.; Yamaji, R.; Fujita, T.; Nakano, Y.; Inui, H. Inhibitive effects of alkyl gallates on hyaluronidase and collagenase. Biosci. Biotechnol. Biochem. 2009, 73, 2335–2337. [Google Scholar]
- Wang, G.; Chen, K.; Chen, L.; Hu, C.; Zhang, D.; Liu, Y. The involvement of the antioxidant system in protection of desert cyanobacterium Nostoc sp. against UV-B radiation and the effects of exogenous antioxidants. Ecotoxicol. Environ. Saf. 2008, 69, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lien, H.M.; Ke, H.J.; Chang, L.L.; Chen, C.C.; Chang, T.M. Antioxidative characteristics of Anisomeles indica extract and inhibitory effect of Ovatodiolode on melanogenesis. Int. J. Mol. Sci. 2012, 13, 6220–6235. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cells Res. 2003, 16, 629–638. [Google Scholar] [CrossRef]
- Tawata, S.; Fukuta, M.; Xuan, T.D.; Deba, F. Total utilization of tropical plants Leucaena leucocephala and Alpinia zerumbet. J. Pestic. Sci. 2008, 33, 40–43. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Koyama, H.; Tawata, S. Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Essential oils, kava pyrones and phenolic compounds from leaves of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chem. 2007, 103, 486–494. [Google Scholar]
- Chompoo, J.; Upadhyay, A.; Kishimoto, W.; Makise, T.; Tawata, S. Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011, 129, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. HIV-1 integrase and neuraminidase inhibitors from Alpinia zerumbet. J. Agric. Food Chem. 2011, 59, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.B.C.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N.; Criddle, D.N.; Fonteles, M.C. Myorelaxant and antispasmodic effects of the essential oil of Alpinia speciosa on rat ileum. Phytother. Res. 2000, 14, 549–551. [Google Scholar] [CrossRef]
- De Araújo, P.V.S.; Coelho-de-Souza, A.N.; Morais, S.M.; Ferreira, S.C.; Leal-Cardoso, J.H. Antinociceptive effects of the essential oil of Alpinia zerumbet on mice. Phytomedicine 2005, 12, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense, L.F.L.; Leal-Cardoso, J.H.; Duarte, G.P. Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats. Fundam. Clin. Pharmacol. 2003, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Victorio, C.P.; Alviano, D.S.; Alviano, C.S.; Lage, C.L.S. Chemical composition of the fractions of leaf oil of Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm. and antimicrobial activity. J. Pharmacol. 2009, 19, 697–701. [Google Scholar]
- Cavalcanti, E.S.B.; de Morai, S.M.; Lima, M.A.A.; Sanata, E.W.P. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem. Inst. Oswaldo Cruz 2004, 99, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Hotta, M.; Kitahara, T.; Takema, Y. A horse chestnut extract, which induces contraction forces in fibroblasts, is a potent anti-aging ingredient. J. Cosmet. Sci. 2006, 57, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.F.; Chiang, B.H. Stimulating effects of Bacillus subtilis natto-fermented Radix astragali on hyaluronic acid production in human skin cells. J. Ethnopharmacol. 2009, 125, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Nagakawa, H.; Moriwaki, S.; Takema, Y.; Fujimura, T.; Imokawa, G. Inhibition of ultraviolet B-induced winkle formation by an elastase inhibiting herbal extract: Implication for the mechanism underlying elastase-associated winkles. Int. J. Dermatol. 2006, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [PubMed]
- Juergens, U.R.; Racke, K.; Tuleta, I.; Skowasch, D.; Stober, M.; Gillissen, A.; Nickenig, G. Antioxidative effects of monoterpenes (1,8-Cineol) compared with budesonide (BUD) on superoxide (O2−) production in human monocytes: New evidence for co-medication in COPD and sinusitis. Am. J. Respir. Crit. Care Med. 2009, 179, A4584. [Google Scholar] [CrossRef]
- Lee, Y.R.; Noh, M.; Kwon, K.B.; Lee, Y.S.; Chu, J.P.; Kim, E.J.; Park, Y.S.; Kim, B.S.; Kim, J.S. Radix clematidis extract inhibits UVB-induced MMP expression by suppressing the NF-κB pathway in human dermal fibroblasts. Int. J. Mol. Med. 2009, 23, 679–684. [Google Scholar] [PubMed]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Park, E.; Kim, J.; Hong, S.; Park, J.; Park, S.; Lee, S.; et al. Glycyrrhizin induces melanogenesis by elevating a cAMP level in B16 melanoma cells. J. Investig. Dermatol. 2005, 124, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.; Taira, N.; Tawata, S. Several herbal compounds in Okinawa plants directly inhibit the oncogenic/aging kinase PAK1. Drug Discov. Ther. 2014, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H. Herbal therapeutics that block the oncogenic kinase PAK1: A practical approach towards PAK1-dependent diseases and longevity. Phytother. Res. 2014, 28, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Be Tu, P.T.; Nguyen, B.C.Q.; Tawata, S.; Yun, C.Y.; Kim, E.G.; Maruta, H. PAK1, p21 (RAC/CDC42)-activated kinase 1, essential for melanogenesis in skin. J. Dermatol. Sci. 2015, in press. [Google Scholar]
- Boskou, G.; Salta, F.N.; Chrysotomou, S.; Mylona, A.; Chiou, A.; Andrikopoulos, N.K. Antioxidant capacity and phenolic profile of table olives from the Greek market. J. Food Chem. 2006, 94, 558–564. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolonization assay. J. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Tripathy, B.C. Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiol. 1992, 98, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Vijayakumar, M.; Rao, C.V.; Shirwaikar, A.; Rawat, A.K.S.; Mehrotra, S.; Pushpangadan, P. Antioxidant potential of Anogeissus latifolia. Biol. Pharm. Bull. 2004, 27, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Steinbrink, D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef]
- Kraunsoe, J.A.E.; Claridge, T.D.W.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Byun, J.C.; Bandi, A.K.R.; Hyun, C.G.; Lee, N.H. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J. Med. Plant Res. 2009, 3, 914–920. [Google Scholar]
- Tadtong, S.; Viriyaroj, A.; Vorarat, S.; Nimkulrat, S.; Suksamrarn, S. Antityrosinase and antibacterial activities of mangosteen pericarp extract. J. Health Res. 2009, 23, 99–102. [Google Scholar]
- Sahgal, G.; Ramanathan, S.; Sasidharan, S.; Mordi, M.N.; Ismail, S.; Mansor, S.M. In vitro antioxidant and xanthine oxidase inhibitory activities of methanolic Swietenia mahagoni seed extracts. Molecules 2009, 14, 4476–4485. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; da Silva Horinouchi, C.D.; da Silveira Prudente, A.; Cechinel-Filho, V.; de Almeida Cabrini, D.; Otuki, M.F. Effect of a Garcinia gardneriana (Planchon and Triana) Zappi hydroalcoholic extract on melanogenesis in B16F10 melanoma cells. J. Ethopharmacol. 2013, 148, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of Phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of tairin and shima EOs are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, P.T.B.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723-16740. https://doi.org/10.3390/molecules200916723
Tu PTB, Tawata S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules. 2015; 20(9):16723-16740. https://doi.org/10.3390/molecules200916723
Chicago/Turabian StyleTu, Pham Thi Be, and Shinkichi Tawata. 2015. "Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet" Molecules 20, no. 9: 16723-16740. https://doi.org/10.3390/molecules200916723






