Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effects of the Total Quercus cortex Extract in an Agar-Diffusion Assay
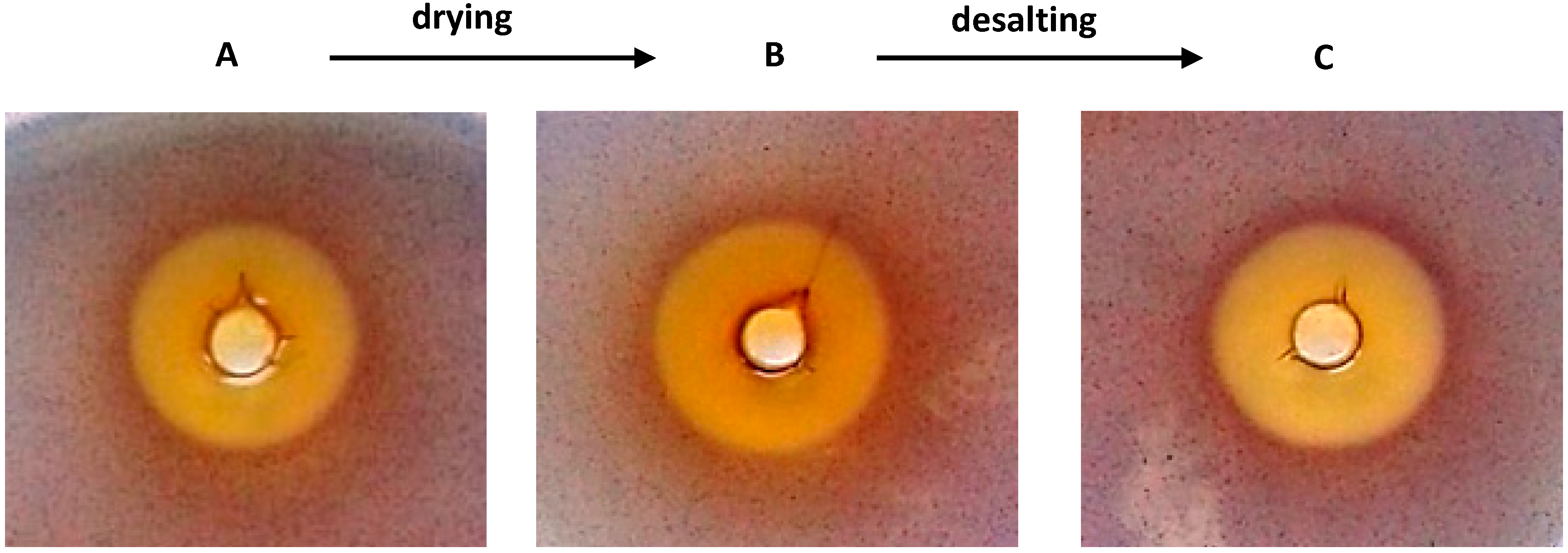
2.2. Reverse Phase High Performance Liquid Chromatography (RP-HPLC) Analysis of Quercus cortex Extract and Screening of Fractions
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Quercus cortex Extract and Compound Library
| No. | Name of the Identified Compounds (IUPAC) | Retention Time, min | Peak Area, % |
|---|---|---|---|
| 1 | propane-1,2,3-triol * | 2.590 | 3.56 |
| 2 | decane * | 2.885 | 0.30 |
| 3 | furan-2-carboxylic acid * | 3.250 | 0.30 |
| 4 | 1,3,5-triazine-2,4,6-triamine * | 3.500 | 0.59 |
| 5 | pentadecane * | 3.755 | 0.25 |
| 6 | 2,3-dihydroxypropanal * | 3.995 | 0.35 |
| 7 | butanedioic acid * | 4.015 | 0.30 |
| 8 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one * | 4.170 | 1.19 |
| 9 | 2-amino-9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one * | 4.270 | 0.59 |
| 10 | cyclopentane-1,2-diol ** | 4.385 | 0.30 |
| 11 | 1,2: 5,6-dianhydrogalactitol ** | 4.695 | 0.89 |
| 12 | 5-(hydroxymethyl)-2-furaldehyde * | 4.825 | 1.98 |
| 13 | (R)-2-acetamido-3-sulfanylpropanoic acid * | 4.955 | 0.89 |
| 14 | 2-propenoic acid, 1-methylundecyl ester ** | 4.995 | 1.39 |
| 15 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one ** | 5.175 | 0.79 |
| 16 | 1-(2-hydroxyethyl)-4-methylpiperazine ** | 5.750 | 1.33 |
| 17 | 6-(4-hydroxy-6-methoxy-2-methyl-tetrahydro-pyran-3-yloxy)-2-methyl-dihydro-pyran-3-one ** | 5.890 | 0.79 |
| 18 | 1,2,3-benzenetriol * | 5.965 | 0.99 |
| 19 | 2-methyl-5-nitro-pyrimidine-4,6-diol ** | 5.975 | 0.99 |
| 20 | 4-hydroxy-3-methoxybenzaldehyde * | 6.260 | 0.53 |
| 21 | 2-amino-9-[3,4-dihydroxy-5- (hydroxymethyl)oxolan-2-yl]-3H-purin-6-one * | 6.445 | 25.6 |
| 22 | 1,6-anhydro-β-d-glucopyranose * | 6.775 | 6.14 |
| 23 | 1- (β-d-arabinofuranosyl)-4-O-trifluoromethyl uracil ** | 7.185 | 0.99 |
| 24 | 4-hydroxy-3-methoxybenzoic acid ** | 7.255 | 0.69 |
| 25 | 1,6-anhydro-β-d-glucofuranose * | 7.400 | 0.89 |
| 26 | 4-propyl-1,3-benzenediol * | 7.525 | 1.38 |
| 27 | cyclohexane-1,2,3,4,5-pentol * | 7.970 | 36.38 |
| 28 | 4-(hydroxymethyl)-2,6-dimethoxyphenol * | 8.225 | 0.37 |
| 29 | 4-(3-hydroxy-1-propenyl)-2-methoxy-phenol * | 8.405 | 4.45 |
| 30 | 9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purine-2,6-dione * | 9.465 | 0.30 |
| 31 | 7-hydroxy-6-methoxy-2H-1-benzopyran-2-one * | 9.545 | 0.48 |
| 32 | methyl-α-d-glucopyranoside * | 9.835 | 1.19 |
| 33 | 2H-1-benzopyran-2-one * | 10.505 | 0.30 |
| 34 | 2-ethoxy-6-(methoxymethyl)phenol ** | 10.935 | 0.75 |
| 35 | 3,4,5-trimethoxy-phenol ** | 13.490 | 1.79 |

2.4. Screening the Library of Compounds in the Growth and Violacein Quantification Assay
| No. | Name of Compound (IUPAC); PubChem CID | Structural Formula | Bioactivity (mM) | |
|---|---|---|---|---|
| MIC | EC50 | |||
| 3 | furan-2-carboxylic acid; 6919 |  | - | - |
| 12 | 5-(hydroxymethyl)-2-furaldehyde; 237332 |  | - | - |
| 18 | 1,2,3-benzenetriol; 1057 |  | 1.7 | 0.175 |
| 20 | 4-hydroxy-3-methoxybenzaldehyde; 1183 |  | - | 0.575 |
| 24 | 4-hydroxy-3-methoxybenzoic acid; 8468 |  | - | - |
| 26 | 4-propyl-1,3-benzenediol; 87874 |  | 0.5 | 0.275 |
| 29 | 4- (3-hydroxy-1-propenyl) -2-methoxy- phenol; 1549095 |  | - | 0.79 |
| 31 | 7-hydroxy-6-methoxy-2H-1-benzopyran-2-one; 5280460 | 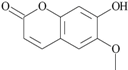 | - | 1.13 |
| 33 | 2H-1-benzopyran-2-one; 323 |  | - | 2.67 |
| 35 | 3,4,5-trimethoxy- phenol; 69505 | 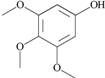 | - | 1 |
| C1 | tetracycline |  | 0.007 | NT |
| C2 | benzo[1,3]dioxol-5-yl amide hexanoic acid |  | NT | 0.052 |

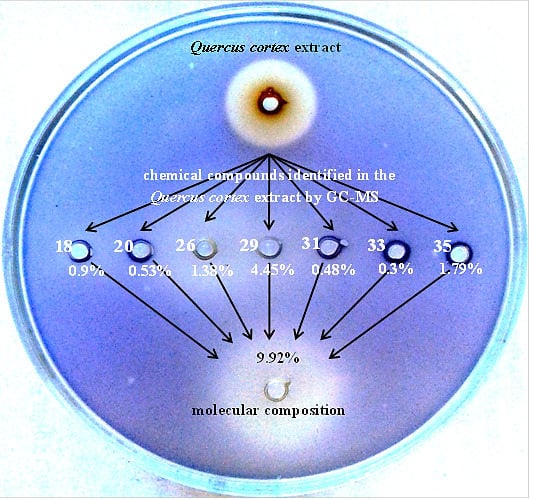
2.5. Reconstruction of Molecular Composition Derived from Quercus cortex Extract

3. Experimental Section
3.1. Plant Material and Extract Preparation
3.2. Reverse Phase High Performance Liquid Chromatography Procedure
3.3. Gas Chromatography-Mass Spectrometry Analysis
3.4. Compound Library
3.5. Bacterial Strain and Culture Conditions
3.6. Bacterial Growth Inhibition and Violacein Production Assays
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Pharmacopoeia, 7th ed.; Supplement 1, Council of Europe: Strasbourg, France, 2011.
- EMEA (European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit). Tylosin: Summary Report (3); Committee for Veterinary Medicinal Products: London, UK, 1997; pp. 205–212. [Google Scholar]
- Gulluce, M.; Sokmen, M.; Sahin, F.; Sokmen, A.; Adiguzel, A.; Ozer, H. Biological activities of the essential oil and methanolic extract of Micromeria fruticosa (L) Druce ssp. Serpyllifolia (Bieb) PH Davis plants from the eastern Anatolia region of Turkey. J. Sci. Food Agric. 2004, 84, 735–741. [Google Scholar]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against Enterohaemorrhagic Escherichia coli O157: H7. J. Ethanopharmacol. 2004, 94, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, S.; Vanmanee, S.; Voravuthikunchai, S. Effect of Thai medicinal plant extracts on cell aggregation of Escherichia coli O157: H7. Songklanakarin J. Sci. Technol. 2005, 27, 545–554. [Google Scholar]
- Andrensek, S.; Simonovska, B.; Vovk, I.; Fyhrquist, P.; Vuorela, H.; Vuorela, P. Antimicrobial and antioxidative enrichment of oak (Quercus robur) bark by rotation planar extraction using ExtraChrom. Int. Food Microbiol. 2004, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.; Kitpipit, L. Activity of medicinal plant extracts against hospital isolates of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2005, 11, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; de Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 25, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.; Ausubel, F.M.; Mathee, K. Medicinal plant extracts attenuate Pseudomonas aeruginosa killing of Caenorhabditis elegans through bacterial virulence inhibition. J. Med. Microbiol. 2008, 57, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, A.A.; Rogozhin, E.A.; Deryabin, D.G. Antibacterial and quorum sensing regulatory activities of some traditional Eastern-European medicinal plants. Acta Pharm. 2014, 64, 173–186. [Google Scholar] [CrossRef]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Vegetable tannins—Lessons of a phytochemical lifetime. Phytochem. 2007, 68, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Kolodziej, H.; Kayser, O.; Latte, K.P.; Ferreira, D. Evaluation of the antimicrobial potency of tannins and related compounds using the microdilution broth method. Planta Med. 1999, 65, 444–446. [Google Scholar] [CrossRef]
- Bacon, J.; Rhodes, M. Binding affinity of hydrolyzable tannins to parotid saliva and to proline-rich proteins derived from it. J. Agric. Food Chem. 2000, 48, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Bennickm, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Mathee, K.; Adonizio, A.L.; Ausubel, F.; Clardy, J.; Bennett, B.; Downum, K. Use of Ellagitannins as Inhibitors of Bacterial Quorum Sensing. WO2009114810 A3, 23 December 2009. [Google Scholar]
- Adonizio, A.; Kong, K.F.; Mathee, K. Inhibition of quorum sensing controlled virulence factor production in Pseudomonas aeruginosa by south Florida plant extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, A.L.; Robison, F.M.; Lyons, S.M.A.; Broeckling, C.D.; Prenni, J.E. Evaluating plant immunity using mass spectrometry-based metabolomics workflows. Front Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ullman, B.; Martin, D.W. Specific cytotoxicity of arabinosylguanine toward cultured T. lymphoblasts. J. Clin. Investig. 1984, 74, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.H.; Zhang, M.S.; Powers, L.S.; Shao, J.Q.; Baltrusaitis, J.; Rutkowski, D.T.; Legge, K.; Monick, M.M. Influenza a viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1). Stress Pathway J. Biol. Chem. 2012, 287, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, F.J.; Kohler, P.R.A.; Rossbach, S. Bacterial inositol catabolism—A sweet ride into the host. Mol. Microb. Ecol. Rhizosphere 2013. [Google Scholar] [CrossRef]
- Kocacalıskan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Verlag Z. Naturforsch C 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Choudhary, G.; Li, M.; Wang, B. Pyrogallol and its analogs can antagonize bacterial quorum sensing in Vibrio harveyi. Bioorg. Med. Chem. Lett. 2008, 18, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Experientia 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, D.G.; Kamayeva, A.A.; Tolmacheva, A.A.; El-Registan, G.I. The effects of alkylhydroxybenzenes on homoserine lactone-induced manifestations of quorum sensing in bacteria. Appl. Biochem. Microbiol. 2014, 50, 353–358. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.; Paul, D.; Kweon, J.H. Inhibition of quorum sensing mechanism and Aeromonas hydrophila biofilm formation by vanillin. Environ. Eng. Sci. 2009, 26, 1359–1363. [Google Scholar] [CrossRef]
- Brencic, A.; Winans, S.C. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol. Mol. Biol. Rev. 2005, 69, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Vouffo, B.; Dongo, E.; Facey, P.; Thorn, A.; Sheldrick, G.; Maier, A.; Fiebig, H.H.; Laatsch, H. Antiarol cinnamate and africanoside, a cinnamoyl triterpene and a hydroperoxy-cardenolide from the stem bark of Antiaris africana. Planta Med. 2010, 6, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef] [PubMed]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.Y.; Kim, S.; Kiriyama, K.; Yoshida, H.; Arai, S.; Ishii, J.; Ogino, C.; Fukuda, H.; Kondo, A. An energy-saving glutathione production method from low-temperature cooked rice using amylase-expressing Saccharomyces cerevisiae. Biotechnol. J. 2012, 7, 686–689. [Google Scholar] [CrossRef] [PubMed]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.S.M.; Tham, F.Y. Anti-quorum sensing and antimicrobial activities of some traditional Chinese medicinal plants commonly used in South-East Asia Sandy. Malays. J. Microbiol. 2012, 8, 11–20. [Google Scholar]
- Deryabin, D.G.; Tolmacheva, A.A. The Use of 1,3-Benzodioxole as Regulators of Collective Behavior (Quorum Sensing) in Bacteria. RU2514001 C2, 10 February 2014. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabin, D.G.; Tolmacheva, A.A. Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract. Molecules 2015, 20, 17093-17108. https://doi.org/10.3390/molecules200917093
Deryabin DG, Tolmacheva AA. Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract. Molecules. 2015; 20(9):17093-17108. https://doi.org/10.3390/molecules200917093
Chicago/Turabian StyleDeryabin, Dmitry G., and Anna A. Tolmacheva. 2015. "Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract" Molecules 20, no. 9: 17093-17108. https://doi.org/10.3390/molecules200917093
APA StyleDeryabin, D. G., & Tolmacheva, A. A. (2015). Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract. Molecules, 20(9), 17093-17108. https://doi.org/10.3390/molecules200917093