Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents
Abstract
:1. Introduction


2. Results and Discussion





| Compound ID | IC50 ± SEM (µM) | ||||
|---|---|---|---|---|---|
| A375 | WM164 | MDA-MB-435 | SJG2 | MT330 | |
| 1a | 6.4 ± 0.8 | 7.5 ± 1.2 | 7.4 ± 0.8 | > 30 | > 30 |
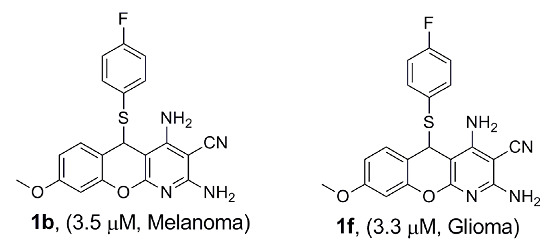
| 1b | 6.7 ± 1.5 | 3.5 ± 1.2 | 3.7 ± 0.8 | 4.7 ± 5 | 6.1 ± 1.9 |
| 1c | 6.3 ± 0.7 | 3.6 ± 0.6 | 4.1 ± 0.4 | 5.6 ± 0.8 | 5.6 ± 0.8 |
| 1d | 7.0 ± 0.9 | 6.6 ± 1.0 | 6.5 ± 0.6 | 5.2 ± 1.4 | 4.6 ± 0.1 |
| 1e | 5.3 ± 0.7 | 5.7 ± 1.4 | 6.0 ± 0.8 | 3.1 ± 2.8 | 4.5 ± 0.9 |
| 1f | 5.7 ± 0.4 | 5.6 ± 0.6 | 7.1 ± 0.5 | 3.3 ± 2.7 | 5.2 ± 0.0 |
| 1g | 7.2 ± 1.4 | 9.7 ± 2.7 | 9.7 ± 2.6 | 5.1 ± 0.1 | 5.4 ± 0.0 |
| 1h | 16.8 ± 0.4 | 25.9 ± 3.5 | 19.1 ± 1.3 | 5.3 ± 0.2 | 8.3 ± 0.0 |
| 1i | 10.6 ± 0.6 | 15.0 ± 2.2 | 8.9 ± 0.7 | 5.1 ± 0.5 | 5.1 ± 0.0 |
| 1j | >30 | >30 | 21.9 ± 4.4 | ND | ND |
| 1k | >30 | >30 | >30 | ND | ND |
| 1l | >30 | >30 | >30 | ND | ND |
| 1m | >30 | >30 | >30 | ND | ND |
| SP-6-27 | 0.08 ± 0.01 | 0.16 ± 0.03 | ND | 0.07 ± 0.02 | 0.05 ± 0.03 |
| Colchicine | 0.02 ± 0.01 | 0.03 ± 0.02 | ND | NT | NT |


3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for the Synthesis of 5H-Substituted-Thiochromenopyridines (1a–1f) under Regular Reflux
3.1.3. General Procedure for the Synthesis of 5H-Substituted-Thiochromenopyridines (1g–1i)
3.1.4. General Procedure for the Synthesis of 5H-Substituted-Thiochromenopyridines (1a–1i) under Microwave Irradiation
3.1.5. General Procedure for the Preparation of 4H-Substituted-Thiochromenes (1k–1m)
3.2. Biology
Cell Culture and Cytotoxicity Assay
Melanoma
Glioma
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Wang, J.; Li, X.S.; Chen, J.; Jones, T.S.; Hosni-Ahmed, A.; Patil, R.; Seibel, W.L.; Li, W.; Miller, D.D. New substituted 4H-chromenes as anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 4458–4461. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Pfeffer, L.M.; Miller, D.D. Gliomas: Classification, Symptoms, Treatment and Prognosis; Nova Science Pub Inc: Hauppauge, NY, USA, 2014. [Google Scholar]
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Wang, Y.; Zhao, J.; Jia, S.; Herich, J.; Labreque, D.; Storer, R.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure-activity relationships of the 4-aryl group. J. Med. Chem. 2004, 47, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H.; Zhao, J.; Jia, S.; Xu, L.; Crogan-Grundy, C.; Denis, R.; Barriault, N.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, A.M.; Litvinov, Y.M.; Rodinovskaya, L.A.; Malyshev, O.R.; Semenova, M.N.; Semenov, V.V. Polyalkoxy Substituted 4H-Chromenes: Synthesis by Domino Reaction and Anticancer Activity. ACS Comb. Sci. 2012, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.X.; et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol. Cancer Ther. 2004, 3, 1375–1384. [Google Scholar] [PubMed]
- Azizmohammadi, M.; Khoobi, M.; Ramazani, A.; Emami, S.; Zarrin, A.; Firuzi, O.; Miri, R.; Shafiee, A. 2H-chromene derivatives bearing thiazolidine-2,4-dione, rhodanine or hydantoin moieties as potential anticancer agents. Eur. J. Med. Chem. 2013, 59, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Khodarahmi, R.; Foroumadi, A.; Mostafaie, A.; Mohammadi Motlagh, H. Anti-angiogenic/proliferative behavior of a “4-aryl-4H-chromene” on blood vessel’s endothelial cells: A possible evidence on dual “anti-tumor” activity. Med. Chem. Res. 2011, 20, 920–929. [Google Scholar] [CrossRef]
- Hussain, M.K.; Ansari, M.I.; Yadav, N.; Gupta, P.K.; Gupta, A.K.; Saxena, R.; Fatima, I.; Manohar, M.; Kushwaha, P.; Khedgikar, V.; et al. Design and synthesis of ERα/ERβ selective coumarin and chromene derivatives as potential anti-breast cancer and anti-osteoporotic agents. RSC Adv. 2014, 4, 8828–8845. [Google Scholar] [CrossRef]
- Thapa, U.; Thapa, P.; Karki, R.; Yun, M.; Choi, J.H.; Jahng, Y.; Lee, E.; Jeon, K.H.; Na, Y.; Ha, E.M.; et al. Synthesis of 2,4-diaryl chromenopyridines and evaluation of their topoisomerase I and II inhibitory activity, cytotoxicity, and structure—activity relationship. Eur. J. Med. Chem. 2011, 46, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, M.; Ghorab, M.; Nissan, Y. Dapson in heterocyclic chemistry, part VIII: Synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1,3-dihydropyridine, chromene and chromenopyridine moieties. Chem. Cent. J. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Hegde, S.; Reinhard, E.; Gomez, L.; Vernier, W.F.; Lee, L.; Liu, S.; Sambandam, S.; Snider, P.A.; Masih, L. Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2005, 15, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Melekhin, E.A.; Bardasov, I.N.; Ershov, O.V.; Eremkin, A.V.; Kayukov, Y.S.; Nasakin, O.E. Synthesis of 5-Aryl-2,4-diamino-8-hydroxy-5H-chromeno[2,3-b]pyridine-3-carbonitriles. Russ. J. Org. Chem. 2006, 42, 622–623. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-Step Synthesis of Heterocyclic Privileged Medicinal Scaffolds by a Multicomponent Reaction of Malononitrile with Aldehydes and Thiols. J. Org. Chem. 2007, 72, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Safaei-Ghomi, J.; Kiani, M.; Ziarati, A.; Shahbazi-Alavi, H. Highly efficient synthesis of benzopyranopyridines via ZrP2O7 nanoparticles catalyzed multicomponent reactions of salicylaldehydes with malononitrile and thiols. J. Sulfur Chem. 2014, 35, 450–457. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Shahbazi-Alavi, H.; Heidari-Baghbahadorani, E. SnO nanoparticles as an efficient catalyst for the one-pot synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines. RSC Adv. 2014, 4, 50668–50677. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, S.; Wang, J.; Lu, Y.; Dalton, J.T.; Miller, D.D.; Li, W. Discovery of novel 2-aryl-4-benzoyl-imidazole (ABI-III) analogues targeting tubulin polymerization as antiproliferative agents. J. Med. Chem. 2012, 55, 7285–7289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Wang, J.; Ahn, S.; Li, C.M.; Lu, Y.; Loveless, V.S.; Dalton, J.T.; Miller, D.D.; Li, W. Novel tubulin polymerization inhibitors overcome multidrug resistance and reduce melanoma lung metastasis. Pharm. Res. 2012, 29, 3040–3052. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1a–1m are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Wang, J.; Pfeffer, S.; Ma, D.; Pfeffer, L.M.; Patil, S.A.; Li, W.; Miller, D.D. Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents. Molecules 2015, 20, 17152-17165. https://doi.org/10.3390/molecules200917152
Banerjee S, Wang J, Pfeffer S, Ma D, Pfeffer LM, Patil SA, Li W, Miller DD. Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents. Molecules. 2015; 20(9):17152-17165. https://doi.org/10.3390/molecules200917152
Chicago/Turabian StyleBanerjee, Souvik, Jin Wang, Susan Pfeffer, Dejian Ma, Lawrence M. Pfeffer, Shivaputra A. Patil, Wei Li, and Duane D. Miller. 2015. "Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents" Molecules 20, no. 9: 17152-17165. https://doi.org/10.3390/molecules200917152