Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
| Substrate (5 mM) | O2 Consumption (mg/L min) | |
|---|---|---|
| Tyrosinase | Laccase | |
| Resveratrol | 3.24 | 2.29 |
| Polydatin | 1.14 | 2.95 |
| Coumaric acid | 0.46 | 0.26 |
| Caffeic acid | 33.10 | 2.86 |
| Ferulic acid | 0.44 | 2.61 |
| Gallic acid | 0.92 | 1.64 |
| Phlorizin | 2.16 | 0.18 |

2.1. Gallic Acid and Caffeic Acid

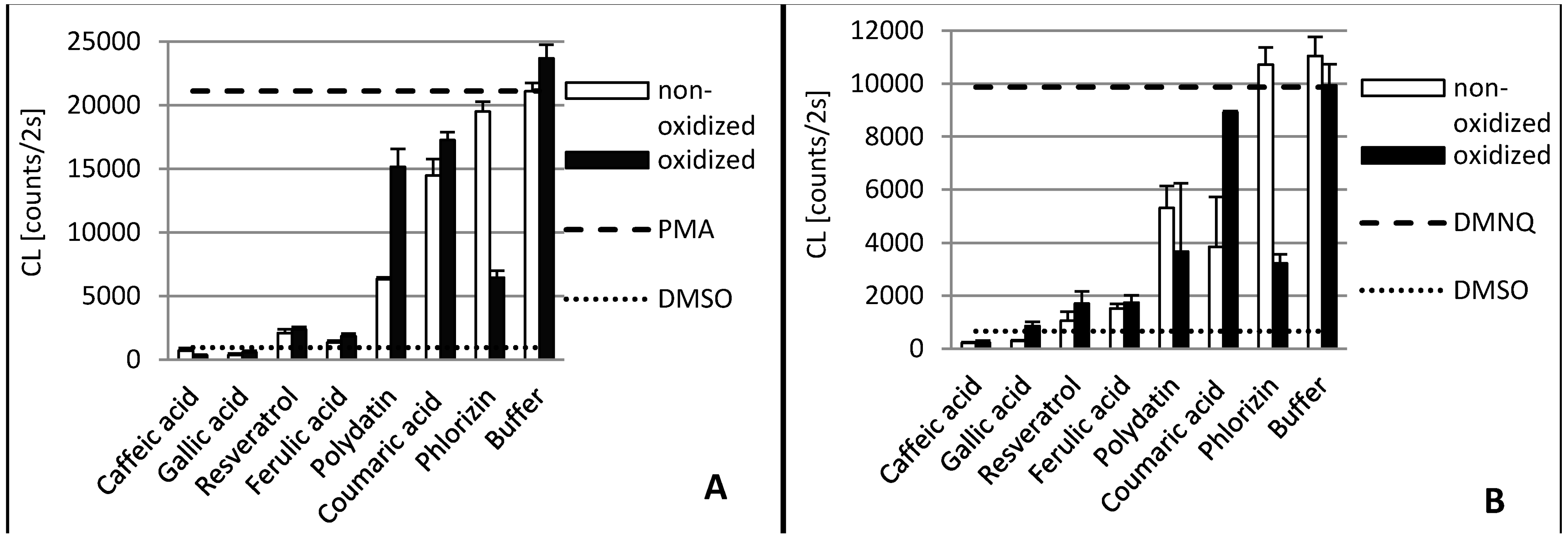

2.2. Ferulic Acid
2.3. p-Coumaric Acid
2.4. Resveratrol and Polydatin
2.5. Phlorizin
3. Experimental Section
3.1. Chemicals
3.2. Polyphenol Oxidase Activity
3.2.1. Spectrophotometric Determination of Activity
3.2.2. Oxygen Consumption Assay
3.3. Determination of Antioxidant Activities of Phenolic Compounds and Their Oxidation Products
3.3.1. Sample Preparation
3.3.2. Chemical Assay for Scavenger Activities with the DPPH• Method
3.3.3. Biological Antioxidant Assay Using Cell Cultures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rec. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar]
- Hosu, A.; Cristea, V.M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Evidence for health benefits of plant phenols: Local or systemieffects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.S.O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymatic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Sureda, A. Editorial: Dietary polyphenols: Well beyond the antioxidant capacity. Cur. Pharm. Biotechnol. 2014, 15, 297. [Google Scholar] [CrossRef]
- Bollman, F.; Art, J.; Henke, J.; Schrick, K.; Besche, V.; Bros, M.; Li, H.; Siuda, D.; Handler, N.; Bauer, F.; et al. Resveratrol post-transcriptionally regulates pro-inflammatory gene expression via regulation of KSRP RNA binding activity. Nucleic Acids Res. 2014, 42, 12555–12569. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Lustrato, G.; Macciola, V.; Ranalli, G. Application of chemical and physical agents in model systems to controlling phenoloxidase enzymes. Eur. Food Res. Technol. 2010, 231, 603–610. [Google Scholar] [CrossRef]
- Macheix, J.J.; Sapis, J.C.; Fleuriet, A. Phenolic compounds and polyphenoloxidase in relation to browning in grapes and wines. Crit. Rev. Food Sci. Nutr. 1991, 30, 441–486. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.J.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–199. [Google Scholar] [CrossRef]
- Claus, H.; Sabel, A.; König, H. Wine Phenols and Laccase: An Ambivalent Relationship. In Wine: Phenolic Composition, Classification and Health Benefits; El Rayess, Y., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 155–185. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microbiol. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, R.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Marusek, C.M.; Trobaugh, N.M.; Flurkey, W.H.; Inlow, J.K. Comparative analysis of polyphenol oxidase from plant and fungal species. J. Inorg. Biochem. 2006, 100, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Gautam, S.G.; Sharma, A. Free phenolics and polyphenol oxidase (PPO): The factors affecting post-cut browning in eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rolff, M.; Schottenheim, J.; Decker, H.; Tuczek, F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011, 40, 4077–4098. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Schweikardt, T.; Tuczek, F. The first crystal structure of tyrosinase: All questions answered? Angew. Chem. Int. Ed. Engl. 2006, 45, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Claus, H. Copper-Containing Oxidases: Occurrence in Soil Microorganisms, Properties and Applications. In Soil Heavy Metals; Sherameti, I., Varma, A., Eds.; Springer-Verlag: Heidelberg, Germany, 2010; pp. 281–313. [Google Scholar]
- González-Santoyom, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of insect immune system. Entmol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Espín, J.C.; Wichers, H.J. Study of the oxidation of resveratrol catalyzed by polyphenol oxidase. Effect of polyphenol oxidase, laccase and peroxidase on the antiradical capacity of resveratrol. J. Food Biochem. 2000, 24, 225–250. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Parker, A.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J. Mol. Catal. B Enzym. 2012, 74, 29–35. [Google Scholar] [CrossRef]
- El Rayess, Y.; Barbar, R.; Wilson, E.A.; Bouajila, J. Analytical Methods for Wine Polyphenols Analysis and for Their Antioxidant Activity Evaluation. In Wine: Phenolic Composition, Classification and Health Benefits; El Rayess, Y., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 71–101. [Google Scholar]
- Li, H.; Horke, S.; Förstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic. Biol. Med. 2009, 47, 1673–1706. [Google Scholar] [PubMed]
- Venugopal, S.K.; Devaraj, S.; Yang, T.; Jialal, I. α-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-α. Diabetes 2002, 51, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Kugelman, A.; Iwamoto, T.; Tian, L.; Forman, H.J. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J. Biol. Chem. 1994, 269, 26512–26517. [Google Scholar] [PubMed]
- Rice-Evans, C.A.; Miller, J.M.; Paganga, G. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food. Agric. 1998, 79, 270–276. [Google Scholar] [CrossRef]
- Nicotra, S.; Cramarossa, M.R.; Mucci, A.; Pagnoni, U.M.; Riva, S.; Forti, L. Biotransformation of resveratrol: Synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophthora thermophyla and from Trametes pubescens. Tetrahedron 2004, 60, 595–600. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, S.Z.; Hudson, G.J.F. Polyhydrochalcones and flavanones as antioxidants for edible oils. Food Chem. 1983, 12, 205–212. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol. Biosci. 2003, 3, 758–764. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Xu, F. Oxidation of phenols, anilines and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Filip, Z. Behaviour of phenoloxidases in the presence of clays and other soil-related absorbents. Appl. Microbiol. Biotechnol. 1988, 28, 506–511. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 18, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Daiber, A.; August, M.; Baldus, S.; Wendt, M.; Oelez, M.; Sydow, K.; Kleschyov, A.L.; Munzel, T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 2004, 36, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar] [PubMed]
- Qin, Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012, 221, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: All chemicals and enzymes used were purchased from external suppliers as specified in the experimental section.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riebel, M.; Sabel, A.; Claus, H.; Fronk, P.; Xia, N.; Li, H.; König, H.; Decker, H. Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines. Molecules 2015, 20, 17194-17207. https://doi.org/10.3390/molecules200917194
Riebel M, Sabel A, Claus H, Fronk P, Xia N, Li H, König H, Decker H. Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines. Molecules. 2015; 20(9):17194-17207. https://doi.org/10.3390/molecules200917194
Chicago/Turabian StyleRiebel, Matthias, Andrea Sabel, Harald Claus, Petra Fronk, Ning Xia, Huige Li, Helmut König, and Heinz Decker. 2015. "Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines" Molecules 20, no. 9: 17194-17207. https://doi.org/10.3390/molecules200917194
APA StyleRiebel, M., Sabel, A., Claus, H., Fronk, P., Xia, N., Li, H., König, H., & Decker, H. (2015). Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines. Molecules, 20(9), 17194-17207. https://doi.org/10.3390/molecules200917194






