Sorption of Cu(II) Ions on Chitosan-Zeolite X Composites: Impact of Gelling and Drying Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Chitosan and Chitosan-Zeolite Composites

| Sample | Zeolite/Chitosan Mass Ratio | Cell Parameter (nm) | Zeolite X | Crystallite Size (nm) | |
|---|---|---|---|---|---|
| Synthesis | Composite | Si/Al | |||
| Zeolite X | 2.497 | 1.29 | 95 | ||
| Composite A | 0.3 | 0.27 | 2.497 | 1.30 | 25 |
| Composite B | 1.0 | 0.94 | 2.498 | 1.27 | 38 |
| Composite C | 1.5 | 1.45 | 2.500 | 1.24 | 35 |


| Sample | Zeolite Mass Fraction | Surface Area (m2·g−1) | Micropore Volume (cm3·g−1) | Mesopore Volume (cm3·g−1) |
|---|---|---|---|---|
| Chitosan aerogel | 0 | 230 | 0 | 0.63 |
| Composite A aerogel | 0.21 | 390 | 0 | 0.89 |
| Composite B aerogel | 0.48 | 360 | 0.04 | 0.63 |
| Composite C aerogel | 0.59 | 480 | 0.11 | 0.44 |
| Zeolite X | 1 | 450 | 0.18 | 0.01 |
| Chitosan xerogel | 0 | 4 | 0 | 0.004 |
| Composite B xerogel | 0.48 | 1 | 0 | 0.002 |
| Composite C xerogel | 0.59 | 4 | 0 | 0.006 |


2.2. Adsorption of Copper
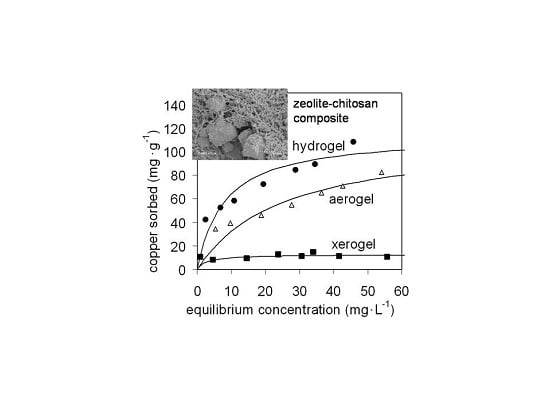
2.2.1. Copper Sorption on Chitosan Gels

| Sample | Zeolite Mass Fraction | Hydrogel | Aerogel | Xerogel | |||
|---|---|---|---|---|---|---|---|
| Qmax (mg·g−1) | K (L·mg−1) | Qmax (mg·g−1) | K (L·mg−1) | Qmax (mg·g−1) | K (L·mg−1) | ||
| Chitosan | 1 | 190% ± 10% | 0.021% ± 18% | 139% ± 9% | 0.016% ± 16% | 24% ± 23% | 0.07% ± 72% |
| Composite A | 0.21 | 155% ± 10% | 0.083% ± 30% | 168% ± 18% | 0.025% ± 33% | 9.4% ± 14% | 0.29% ± 63% |
| Composite B | 0.48 | 111% ± 16% | 0.15% ± 62% | 113% ± 14% | 0.040% ± 34% | 12.7% ± 9% | 0.38% ± 72% |
| Composite C | 0.59 | 83% ± 9% | 0.28% ± 50% | 110% ± 13% | 0.065% ± 38% | 15.6% ± 10% | 3.5% ± 100% |
2.2.2. Sorption of Cu2+ on Zeolite Na-X
2.2.3. Sorption Isotherms of Cu2+ on Composites Chitosan-Zeolite X

3. Experimental Section
3.1. Materials
3.2. Preparation of Chitosan Beads
3.3. Preparation of Chitosan-Zeolite Composites
3.4. Characterization of Materials
3.5. Copper Sorption Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chassary, P.; Vincent, T.; Guibal, E. Metal anion sorption on chitosan and derivative materials: A strategy for polymer modification and optimum use. React. Funct. Polym. 2004, 60, 137–149. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Laus, R.; Geremias, R.; Vasconcelos, H.L.; Laranjeira, M.C.M.; Favere, V.T. Reduction of acidity and removal of metal ions from coal mining effluents using chitosan microspheres. J. Hazard. Mater. 2007, 149, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Gschaider, M.E. Theoretical and experimental study of Pb2+ and Hg2+ adsorption on biopolymers, 1. Theoretical study. Macromol. Biosci. 2001, 1, 233–248. [Google Scholar] [CrossRef]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Removal of selected metal ions from aqueous solutions using chitosan flakes. Sep. Sci. Technol. 2000, 35, 547–560. [Google Scholar] [CrossRef]
- Juang, R.S.; Shao, H.J. A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res. 2002, 36, 2999–3008. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; Macmillan: Basingstoke, UK, 1992. [Google Scholar]
- Vårum, K.M.; Smidsrød, O. Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor and Francis: Boca Raton, FA, USA, 2006; pp. 497–520. [Google Scholar]
- Tolaimate, A.; Desbrieres, J.; Rhazia, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Canh, L.T.; Lacroix, M.; Szabo, P.I.; Mateescu, M.A. Seafood Quality and Safety: Advances in the New Millennium; Shaidi, F., Simpson, B., Eds.; DEStech: Lancaster, PA, USA, 2004; pp. 251–274. [Google Scholar]
- Mullin, R. Basic materials keep a technology edge. Chem. Eng. News 2002, 80, 44–48. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Macquarrie, D.J.; Hardy, J.J.E. Applications of functionalized chitosan in catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Drogoz, A.; David, L.; Domard, A.; Charles, M.H.; Verrier, B.; Delair, T. Polysaccharide-based vaccine delivery systems: Macromolecular assembly, interactions with antigen presenting cells, and in vivo immunomonitoring. J. Biomed. Res. A 2010, 93, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.; Tobin, J.M.; Guibal, E. Influence of chitosan preprotonation on reactive black 5 sorption isotherms and kinetics. Ind. Eng. Chem. Res. 2004, 43, 1–11. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using bath studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Quignard, F.; Choplin, A.; Domard, A. Chitosan: A natural polymeric support of catalysts for the synthesis of fine chemicals. Langmuir 2000, 162, 9106–9108. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized chitosan as a green, recyclable, biopolymer-supported catalyst for the [3 + 2] Huisgen cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, A.; Crucianelli, M.; Passacantando, M.; Nisi, S.; Saladino, R. Chitin- and chitosan-anchored methyltrioxorhenium: An innovative approach for selective heterogeneous catalytic epoxidations of olefins. J. Catal. 2010, 276, 412–422. [Google Scholar] [CrossRef]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan aerogel: A recyclable, heterogeneous organocatalyst for the asymmetric direct aldol reaction in water. Chem. Commun. 2010, 46, 6288–6290. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shalom, N.; Fallik, E. Further suppression of Botrytis cinerea disease in cucumber seedlings by chitosan-copper complex as compared with chitosan alone. Phytoparasitica 2003, 31, 99–102. [Google Scholar] [CrossRef]
- Valentin, R.; Molvinger, K.; Quignard, F.; Brunel, D. Supercritical CO2 dried chitosan: An efficient intrinsic heterogeneous catalyst in fine chemistry. New J. Chem. 2003, 27, 1690–1692. [Google Scholar] [CrossRef]
- Valentin, R.; Bonelli, B.; Garrone, E.; Di Renzo, F.; Quignard, F. Accessibility of the functional groups of chitosan aerogel probed by FT-IR-monitored deuteration. Biomacromolecules 2007, 8, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Chen, D.; Jiao, X. Chitosan-based aerogels with high adsorption performance. J. Phys. Chem. B 2008, 112, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yu, B.; Yue, Z.; Wang, T.; Wen, X.; Liu, Z.; Zhao, C. Preparation of porous chitosan gel beads for copper(II) ion adsorption. J. Hazard. Mater. 2007, 147, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H. Removal of copper from aqueous solution by chitosan in prawn shell: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2002, 90, 77–95. [Google Scholar] [CrossRef]
- Krajewska, B.; Olech, A. Pore structure of gel chitosan membranes. I. Solute diffusion measurements. Polym. Gels Netw. 1996, 4, 33–43. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Quignard, F.; Di Renzo, F.; Guibal, E. From natural polysaccharides to materials for catalysis, adsorption, and remediation. Top. Curr. Chem. 2010, 294, 165–197. [Google Scholar] [PubMed]
- Peirano, F.; Vincent, T.; Quignard, F.; Robitzer, M.; Guibal, E. Palladium supported on chitosan hollow fiber for nitrotoluene hydrogenation. J. Membr. Sci. 2009, 329, 30–45. [Google Scholar] [CrossRef]
- Yu, L.; Gong, J.; Zeng, C.; Zhang, L. Synthesis of binderless zeolite X microspheres and their CO2 adsorption properties. Sep. Purif. Technol. 2013, 118, 188–195. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Muzzarelli, R.A.A. Natural and artificial chitosan–inorganic composites. J. Inorg. Biochem. 2002, 92, 89–94. [Google Scholar] [CrossRef]
- Wan, M.W.; Petrisor, I.G.; Lai, H.T.; Kim, D.; Yen, T.F. Copper adsorption through chitosan immobilized on sand to demonstrate the feasibility for in situ soil decontamination. Carbohydr. Polym. 2004, 55, 249–254. [Google Scholar] [CrossRef]
- Molvinger, K.; Quignard, F.; Brunel, D.; Boissière, M.; Devoisselle, J.M. Porous chitosan-silica hybrid microspheres as a potential catalyst. Chem. Mater. 2004, 16, 3367–3372. [Google Scholar] [CrossRef]
- Primo, A.; Quignard, F. Chitosan as efficient porous support for dispersion of highly active gold nanoparticles: Design of hybrid catalyst for carbon–carbon bond formation. Chem. Commun. 2010, 46, 5593–5595. [Google Scholar] [CrossRef] [PubMed]
- De Lathouder, K.M.; Smeltink, M.W.; Straathof, A.J.J.; Paasman, M.A.; van de Sandt, E.J.A.X.; Kapteijn, F.; Moulijn, J.A. Hydrogel coated monoliths for enzymatic hydrolysis of penicillin G. J. Ind. Microbiol. Biotechnol. 2008, 35, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Tan, W.B.; Zhang, Y. Synthesis and characterization of monodisperse chitosan nanoparticles with embedded quantum dots. Nanotechnology 2006, 17, 140–144. [Google Scholar] [CrossRef]
- Liu, F.; Carlos, L.D.; Ferreira, R.A.S.; Rocha, J.; Ferro, M.C.; Tourrette, A.; Quignard, F.; Robitzer, M. Synthesis, texture, and photoluminescence of lanthanide-containing chitosan−silica hybrids. J. Phys. Chem. B 2010, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Liu, Q.L.; Fang, J.; Zhu, A.M.; Zhang, Q.G. Composite hybrid membrane of chitosan–silica in pervaporation separation of MeOH/DMC mixtures. J. Colloid Interface Sci. 2007, 316, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK, 1978. [Google Scholar]
- 4A Zeolite Molecular Sieve. Available online: http://www.guidechem.com (accessed on 12 June 2015).
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Chi, L.; Wu, D. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel 2013, 103, 480–485. [Google Scholar] [CrossRef]
- Arora, M.; Eddy, N.K.; Mumford, K.A.; Baba, Y.; Perera, J.M.; Stevens, G.W. Surface modification of natural zeolite by chitosan and its use for nitrate removal in cold regions. Cold Reg. Sci. Technol. 2010, 62, 92–97. [Google Scholar] [CrossRef]
- Nešić, A.R.; Veličković, S.J.; Antonović, D.G. Modification of chitosan by zeolite A and adsorption of Bezactive Orange 16 from aqueous solution. Compos. B 2013, 53, 145–151. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Chao, C.; Zhang, B.; Liu, J. In-situ preparation of NaA zeolite/chitosan porous hybrid beads for removal of ammonium from aqueous solution. Carbohydr. Polym. 2014, 107, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V.; Timpu, D. Preparation and characterization of novel composites based on chitosan and clinoptilolite with enhanced adsorption properties for Cu2+. Bioresour. Technol. 2010, 101, 812–817. [Google Scholar]
- Zhang, Y.; Yan, W.; Sun, Z.; Pan, C.; Mi, X.; Zhao, G.; Gao, J. Fabrication of porous zeolite/chitosan monoliths and their applications for drug release and metal ions adsorption. Carbohydr. Polym. 2015, 117, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Wu, H.; Zheng, B. Effect of zeolites on chitosan/zeolite hybrid membranes for direct methanol fuel cell. J. Power Sources 2008, 178, 9–19. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Li, H.; Yang, D. Chitosan membranes filled by GPTMS-modified zeolite beta particles with low methanol permeability for DMFC. Chem. Eng. Process. 2010, 49, 278–285. [Google Scholar] [CrossRef]
- Patil, M.B.; Aminabhavi, T.M. Pervaporation separation of toluene/alcohol mixtures using silicalite zeolite embedded chitosan mixed matrix membranes. Sep. Purif. Technol. 2008, 62, 128–136. [Google Scholar] [CrossRef]
- Liu, B.; Cao, Y.; Wang, T.; Yuan, Q. Preparation of novel ZSM-5 zeolite-filled chitosan membranes for pervaporation separation of dimethyl carbonate/methanol mixtures. J. Appl. Polym. Sci. 2007, 106, 2117–2125. [Google Scholar] [CrossRef]
- Dogan, H.; Hilmioglu, N.D. Chitosan coated zeolite filled regenerated cellulose membrane for dehydration of ethylene glycol/water mixtures by pervaporation. Desalination 2010, 258, 120–127. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mohd Nawawi, M.G.; So, L.K. Characterization and performance evaluations of sodium zeolite-Y filled chitosan polymeric membrane: Effect of sodium zeolite-Y concentration. J. Appl. Polym. Sci. 2006, 99, 1740–1751. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Chen, X.; Jiang, Z. Surface-modified zeolite-filled chitosan membranes for pervaporation dehydration of ethanol. Appl. Surf. Sci. 2008, 254, 5367–5374. [Google Scholar] [CrossRef]
- Varghese, J.G.; Kittur, A.A.; Kariduraganavar, M.Y. Dehydration of THF-water mixtures using zeolite-incorporated polymeric membranes. J. Appl. Polym. Sci. 2009, 111, 2408–2418. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Toh, R.H.; Hanafiah, M.A.K.M. Utilization of chitosan–zeolite composite in the removal of Cu(II) from aqueous solution: Adsorption, desorption and fixed bed column studies. Chem. Eng. J. 2012, 209, 46–53. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Wong, C.S.; Hanafiah, M.A.K.M. Preparation and characterization of chitosan–zeolite composites. J. Appl. Polym. Sci. 2012, 125, 2417–2425. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Toh, R.H.; Hanafiah, M.A.K.M. Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan–zeolite composites. Chem. Eng. J. 2013, 223, 231–238. [Google Scholar] [CrossRef]
- Batista, A.C.L.; Villanueva, E.R.; Amorim, R.V.S.; Tavares, M.T.; Campos-Takaki, G.M. Chromium (VI) ion adsorption features of chitosan film and its chitosan/zeolite conjugate 13X film. Molecules 2011, 16, 3569–3579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, D.; Zheng, X.; Jiang, Z.; Li, J. Zeolite β-filled chitosan membrane with low methanol permeability for direct methanol fuel cell. J. Power Sources 2008, 183, 454–463. [Google Scholar] [CrossRef]
- Yuan, W.; Wu, H.; Zheng, B.; Zheng, X.; Jiang, Z.; Hao, X.; Wang, B. Sorbitol-plasticized chitosan/zeolite hybrid membrane for direct methanol fuel cell. J. Power Sources 2007, 172, 604–612. [Google Scholar] [CrossRef]
- Kittur, A.A.; Kulkarni, S.S.; Aralaguppi, M.I.; Karinuranavagar, M.Y. Preparation and characterization of novel pervaporation membranes for the separation of water–isopropanol mixtures using chitosan and NaY zeolite. J. Membr. Sci. 2005, 247, 75–86. [Google Scholar] [CrossRef]
- Yang, S.; Navrotsky, A.; Phillips, B.L. An in situ calorimetric study of the synthesis of FAU zeolite. Microporous Mesoporous Mater. 2001, 46, 137–151. [Google Scholar] [CrossRef]
- Kosanovic, C.; Subotic, B.; Smit, I. Results of hydrothermal treatment of the amorphous phases obtained by ball milling of zeolites A, X and synthetic mordenite. Croat. Chem. Acta 2001, 74, 195–206. [Google Scholar]
- Dempsey, E.; Kuhl, G.H.; Olson, D.H. Variation of the lattice parameter with aluminum content in synthetic sodium faujasites. Evidence for ordering of the framework ions. J. Phys. Chem. 1969, 73, 387–390. [Google Scholar] [CrossRef]
- Sohn, J.R.; Decanio, S.J.; Lunsford, J.H. Determination of framework aluminium content in dealuminated Y-type zeolites: A comparison based on unit cell size and wavenumber of i.r. bands. Zeolites 1986, 6, 225–227. [Google Scholar] [CrossRef]
- Kerr, G.T. Determination of framework aluminum content in zeolites X, Y, and dealuminated Y using unit cell size. Zeolites 1989, 9, 350–351. [Google Scholar] [CrossRef]
- Caullet, P.; Guth, J.L.; Wey, R. Solubility and thermodynamic constants of dissolution of zeolites 4A and 13X in basic aqueous solutions. Bull. Mineral. 1980, 103, 330–335. [Google Scholar]
- Sefcik, J.; McCormick, A.V. Prediction of crystallization diagrams for synthesis of zeolites. Chem. Eng. Sci. 1999, 54, 3513–3519. [Google Scholar] [CrossRef]
- Bouchiba, N.; Guzman Castillo, M.L.; Bengueddach, A.; Fajula, F.; Di Renzo, F. Zeolite metastability as a function of the composition of the surrounding solution: The case of faujasite and zeolite omega. Microporous Mesoporous Mater. 2011, 144, 195–199. [Google Scholar] [CrossRef]
- Bihari-Varga, M.; Sepulchre, C.; Moczar, E. Chitosan kills bacteria through cell membrane damage. J. Therm. Anal. 1975, 7, 675–683. [Google Scholar] [CrossRef]
- Chabanis, G.; Abdoulaye, A.; Giuntini, J.C.; Zanchetta, J.V.; di Renzo, F.; Vanderschueren, J. Dielectric properties of an NaX zeolite as a function of the hydration state. J. Chem. Soc. Faraday Trans. 1997, 93, 4085–4090. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Valentin, R.; Molvinger, K.; Viton, C.; Domard, A.; Quignard, F. From hydrocolloids to high specific surface area porous supports for catalysis. Biomacromolecules 2005, 6, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Monteiro, O.A.C.; Airoldi, C. Some Thermodynamic data on copper–chitin and copper–chitosan biopolymer interactions. J. Colloid Interface Sci. 1999, 212, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Svilović, S.; Rušić, D.; Stipišić, R. Modeling batch kinetics of copper ions sorption using synthetic zeolite NaX. J. Hazard. Mater. 2009, 170, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.A. The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers. Colloid Surf. A 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Maes, A.; Cremers, A. Ion exchange of synthetic zeolite X and Y with Co2+, Ni2+, Cu2+ and Zn2+ ions. J. Chem. Soc. Faraday Trans. 1975, 1, 265–277. [Google Scholar] [CrossRef]
- Miya, M.; Iwamoto, R.; Yoshikawa, S.; Mima, S.I.R. spectroscopic determination of CONH content in highly deacylated chitosan. Int. J. Biol. Macromol. 1980, 2, 323–324. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H-NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Robitzer, M.; Di Renzo, F.; Quignard, F. Natural materials with high surface area. Physisorption methods for the characterization of the texture and surface of polysaccharide aerogels. Microporous Mesoporous Mater. 2011, 140, 9–16. [Google Scholar] [CrossRef]
- Neimark, A.V.; Ravikovitch, P.I. Capillary condensation in MMS and pore structure characterization. Microporous Mesoporous Mater. 2001, 44, 697–707. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Isa, I.M. Comparison study of copper ion adsorption on chitosan, Dowex A−, and Zerolit 225. J. Appl. Polym. Sci. 1998, 67, 1067–1070. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Hartmann, R.L.; Fogler, H.S. Understanding the dissolution of zeolites. Langmuir 2007, 23, 5477–5484. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the zeolite-chitosan composites are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djelad, A.; Morsli, A.; Robitzer, M.; Bengueddach, A.; Di Renzo, F.; Quignard, F. Sorption of Cu(II) Ions on Chitosan-Zeolite X Composites: Impact of Gelling and Drying Conditions. Molecules 2016, 21, 109. https://doi.org/10.3390/molecules21010109
Djelad A, Morsli A, Robitzer M, Bengueddach A, Di Renzo F, Quignard F. Sorption of Cu(II) Ions on Chitosan-Zeolite X Composites: Impact of Gelling and Drying Conditions. Molecules. 2016; 21(1):109. https://doi.org/10.3390/molecules21010109
Chicago/Turabian StyleDjelad, Amal, Amine Morsli, Mike Robitzer, Abdelkader Bengueddach, Francesco Di Renzo, and Françoise Quignard. 2016. "Sorption of Cu(II) Ions on Chitosan-Zeolite X Composites: Impact of Gelling and Drying Conditions" Molecules 21, no. 1: 109. https://doi.org/10.3390/molecules21010109