Cytotoxic Compounds from Juglans sinensis Dode Display Anti-Proliferative Activity by Inducing Apoptosis in Human Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
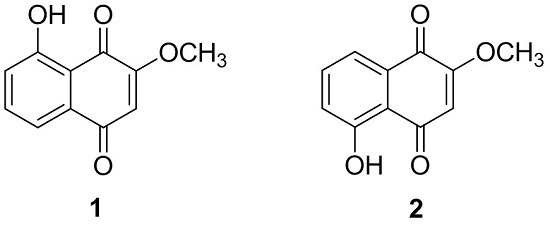
2.1. Phytochemical Characterization of the Bark of J. sinensis


2.2. Biological Evaluations of Compounds
2.2.1. Identification of Cytotoxic Compounds
| Samples | IC50 |
|---|---|
| Methanol extract | 153.4 ± 10.61 a |
| Hexane fraction | 41.92 ± 3.68 a |
| Ethyl acetate fraction | 31.23 ± 0.67 a |
| Butanol fraction | 61.51 ± 2.00 a |
| Aqueous fraction | 155.3 ± 10.71 a |
| 1 | 1.33 b |
| 2 | 1.82 b |
| 3–17 | >10 b |
2.2.2. Cytotoxic Effects of Compounds 1 and 2 against Various Human Cancer Cells

| Compounds | MCF7 | SNU423 | SH-SY5Y | HeLa | HCT116 | A549 |
|---|---|---|---|---|---|---|
| 1 | 1.95 ± 0.05 | 10.87 ± 0.33 | 4.13 ± 0.27 | 4.07 ± 0.06 | 1.83 ± 0.12 | 1.33 ± 0.02 |
| 2 | 1.96 ± 0.04 | 10.42 ± 0.31 | 6.07 ± 0.05 | 3.70 ± 0.05 | 3.00 ± 0.12 | 1.82 ± 0.03 |

2.2.3. Cancer Cell Specific Cytotoxicity of Compounds 1 and 2

2.2.4. Anti-Proliferative Activity of Compounds 1 and 2


2.2.5. Apoptotic Activity of Compounds 1 and 2

2.3. Discussion
3. Experimental Section
3.1. General Procedures
3.2. Materials and Chemicals
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Colony Formation Assay
3.7. Flow Cytometry Analysis
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Yang, H.; Cho, H.-J.; Sim, S.H.; Chung, Y.K.; Kim, D.-D.; Sung, S.H.; Kim, J.; Kim, Y.C. Cytotoxic terpenoids from Juglans sinensis leaves and twigs. Bioorg. Med. Chem. Lett. 2012, 22, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective diarylheptanoids from the leaves and twigs of Juglans sinensis against glutamate-induced toxicity in HT22 cells. Planta Med. 2011, 77, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jeong, E.J.; Kim, J.W.; Sung, S.H.; Kim, Y.C. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 Cells. J. Nat. Prod. 2011, 74, 751–756. [Google Scholar] [CrossRef] [PubMed]
- An, R.-B.; Kim, H.-C.; Tian, Y.-H.; Kim, Y.-C. Free radical scavenging and hepatoprotective constituents from the leaves of Juglans sinensis. Arch. Pharm. Res. 2005, 28, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Kim, S.-H. Immunomodulatory effect of Juglans sinensis, Psoralea corylifolia, cheong-a-hwan extract and cyclosporine A on Th1 (IFN-γ)/Th2 (IL-4) cytokine balance, eosinophil accumulation in a murine model of asthma. Phytochem. Lett. 2008, 1, 6–10. [Google Scholar] [CrossRef]
- Yang, H.; Sung, S.H.; Kim, Y.C. The ethanolic extract of Juglans sinensis leaves and twigs attenuates CCl4-induced hepatic oxidative stress in rats. Pharmacogn. Mag. 2015, 11, 533–539. [Google Scholar] [PubMed]
- Ahn, C.B.; Song, C.H.; Kim, W.H.; Kim, Y.K. Effects of Juglans sinensis Dode extract and antioxidant on mercury chloride-induced acute renal failure in rabbits. J. Ethnopharmacol. 2002, 82, 45–49. [Google Scholar] [CrossRef]
- Hasan, T.N.; B, L.G.; Shafi, G.; Al-Hazzani, A.A.; Alshatwi, A.A. Anti-proliferative effects of organic extracts from root bark of Juglans regia L. (RBJR) on MDA-MB-231 human breast cancer cells: Role of Bcl-2/Bax, caspases and Tp53. Asian Pac. J. Cancer Prev. 2011, 12, 525–530. [Google Scholar] [PubMed]
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol. 2011, 49, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, H.G.; Hong, S.P.; Kim, S.Y.; Oh, M.S. Walnuts (seeds of Juglandis sinensis L.) protect human epidermal keratinocytes against UVB-induced mitochondria-mediated apoptosis through upregulation of ROS elimination pathways. Skin Pharmacol. Physiol. 2014, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, L.; Brassard, P. Regiospecific addition of monooxygenated dienes to halo quinones. J. Org. Chem. 1988, 53, 4052–4059. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, B.; Jiang, Y.; Liu, Z.; Liu, Y.; Wang, X.; Kuang, H. Studies on Cytotoxic Activity against HepG-2 Cells of Naphthoquinones from Green Walnut Husks of Juglans mandshurica Maxim. Molecules 2015, 20, 15572–15588. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, J.; Li, Q.; Zhang, L.; Chen, A. Antifungal constituents from the husk of Carya cathayensis. Linye Kexue 2009, 45, 90–94. [Google Scholar]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar] [CrossRef]
- Machida, K.; Matsuoka, E.; Kasahara, T.; Kikuchi, M. Studies on the constituents of Juglans species. I. Structure determination of (4S)- and (4R)-4-hydroxy-α-tetralone derivatives from the fruit of Juglans mandshurica Maxim. Var. sieboldiana Makino. Chem. Pharm. Bull. 2005, 53, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Li, X.; Meng, F.-Y.; Pi, H.-F.; Zhang, P.; Ruan, H.-L. Naphthoquinones from the root barks of Juglans cathayensis Dode. J. Asian Nat. Prod. Res. 2011, 13, 581–587. [Google Scholar] [CrossRef]
- Jerz, G.; Waibel, R.; Achenbach, H. Cyclohexanoid protoflavanones from the stem-bark and roots of Ongokea gore. Phytochemistry 2005, 66, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Gaffield, W. Circular dichroism, optical rotatory dispersion, and absolute configuration of flavanones, 3-hydroxyflavanones, and their glycosides. Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem. Anal. 2011, 23, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bae, K.H. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Hisama, M. Antimutagenic activity flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Kim, J.M.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.-H.; Kim, J.S. Flavonols from Houttuynia cordata with protein glycation and aldose reductase inhibitory activity. Nat. Prod. Res. 2006, 12, 210–213. [Google Scholar]
- Wu, Z.; Li, R. Chemical constituents from the roots of Rhododendron spiciferum. Tianran Chanwu Yanjiu Yu Kaifa 2011, 23, 253–257. [Google Scholar]
- Jang, K.C.; Lee, J.H.; Kim, S.C.; Song, E.Y.; Ro, N.Y.; Moon, D.Y.; Um, Y.C.; Park, K.H. Antibacterial and radical scavenging activities of 1-C-(p-hydroxyphenyl)-glycerol from Trichosanthes kirilowii. J. Appl. Biol. Chem. 2007, 50, 17–21. [Google Scholar]
- Machida, K.; Yogiashi, Y.; Matsuda, S.; Suzuki, A.; Kikuchi, M. A new phenolic glycoside syringate from the bark of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. J. Nat. Med. 2009, 63, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Al-Howiriny, T.A. Two new hydroxy chalcone derivatives from Thymus cilicicus. Z. Naturforsch. 2007, 62b, 121–124. [Google Scholar] [CrossRef]
- Poppe, L.; Novak, L.; Kajtar-Peredy, M.; Szantay, C. Lipase-catalyzed enantiomer selective hydrolysis of 1,2-diol diacetates. Tetrahedron 1993, 4, 2211–2217. [Google Scholar] [CrossRef]
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, K.S.; Son, J.K.; Je, G.H.; Lee, J.S.; Lee, C.H.; Cheong, C.J. Cytotoxic compounds from the roots of Juglans mandshurica. J. Nat. Prod. 1998, 61, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, Y.; Shang, X.Y.; Cui, B.S.; Yuan, Y.; Chen, X.G.; Yang, Y.C.; Shi, J.G. Triterpenoids from the roots of Pterospermum heterophyllum Hance. J. Asian Nat. Prod. Res. 2009, 11, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Pelageev, D.N.; Dyshlovoy, S.A.; Pokhilo, N.D.; Denisenko, V.A.; Borisova, K.L.; Keller-von Amsberg, G.; Bokemeyer, C.; Fedorov, S.N.; Honecker, F.; Anufriev, V.P. Quinone-carbohydrate nonglucoside conjugates as a new type of cytotoxic agents: Synthesis and determination of in vitro activity. Eur. J. Med. Chem. 2014, 77, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.H.; Yong, P.V.C.; Lim, Y.M.; Ho, A.S.H. 2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways. Life Sci. 2015, 135, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.M.; Pawlowski, K.; Haas, E.; Ware, C.F.; Godzik, A.; Reed, J.C. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J. Biol. Chem. 2001, 276, 24242–24252. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Omoto, T.; Asai, I.; Ezaki, K.; Shimomura, K. Taxifolin 3-arabinoside from Fragaria xananassa. Phytochemistry 1995, 40, 345–347. [Google Scholar] [CrossRef]
- Li, Y.-L.; Gao, Y.-X.; Jin, H.-Z.; Shan, L.; Chang, W.-L.; Yang, X.-W.; Zeng, H.-W.; Wang, N.; Steinmetz, A.; Zang, W.-D. Chemical constituents of Abies fabri. Phytochemistry 2015, 117, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, E.J.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry 2013, 92, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Cui, J.; Lee, J.; Han, A.-R.; Lee, E.B.; Jang, H.H.; Seo, E.K. Cytotoxic Compounds from Juglans sinensis Dode Display Anti-Proliferative Activity by Inducing Apoptosis in Human Cancer Cells. Molecules 2016, 21, 120. https://doi.org/10.3390/molecules21010120
Lee YJ, Cui J, Lee J, Han A-R, Lee EB, Jang HH, Seo EK. Cytotoxic Compounds from Juglans sinensis Dode Display Anti-Proliferative Activity by Inducing Apoptosis in Human Cancer Cells. Molecules. 2016; 21(1):120. https://doi.org/10.3390/molecules21010120
Chicago/Turabian StyleLee, Yoo Jin, Jun Cui, Jun Lee, Ah-Reum Han, Eun Byul Lee, Ho Hee Jang, and Eun Kyoung Seo. 2016. "Cytotoxic Compounds from Juglans sinensis Dode Display Anti-Proliferative Activity by Inducing Apoptosis in Human Cancer Cells" Molecules 21, no. 1: 120. https://doi.org/10.3390/molecules21010120
APA StyleLee, Y. J., Cui, J., Lee, J., Han, A.-R., Lee, E. B., Jang, H. H., & Seo, E. K. (2016). Cytotoxic Compounds from Juglans sinensis Dode Display Anti-Proliferative Activity by Inducing Apoptosis in Human Cancer Cells. Molecules, 21(1), 120. https://doi.org/10.3390/molecules21010120