Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Factor Experiment
2.1.1. Effect of Ethanol Concentration

2.1.2. Effect of Solvent/Material Ratio
2.1.3. Effect of Ultrasound Irradiation Time
2.1.4. Effect of Ultrasound Irradiation Temperature
2.2. Response Surface Methodology
2.2.1. Experimental Design and Results of CCRD
| Run | X1 (Concentration of Ethanol, %) | X2 (Solvent/Material Ratio, mL/g) | X3 (Extraction Time, min) | Y (TEAC Value, µmol Trolox/g DW) |
|---|---|---|---|---|
| 1 | 70 | 50 | 7 | 1082.39 |
| 2 | 83.64 | 40 | 15 | 952.35 |
| 3 | 50 | 40 | 15 | 1063.53 |
| 4 | 50 | 40 | 15 | 1086.78 |
| 5 | 30 | 30 | 23 | 977.83 |
| 6 | 50 | 23.18 | 15 | 951.72 |
| 7 | 50 | 40 | 15 | 1051.91 |
| 8 | 70 | 30 | 7 | 960.39 |
| 9 | 50 | 56.82 | 15 | 1092.03 |
| 10 | 30 | 50 | 23 | 1000.05 |
| 11 | 50 | 40 | 28.45 | 1009.28 |
| 12 | 50 | 40 | 15 | 1040.28 |
| 13 | 50 | 40 | 15 | 1071.28 |
| 14 | 70 | 50 | 23 | 1029.11 |
| 15 | 50 | 40 | 15 | 1055.78 |
| 16 | 70 | 30 | 23 | 948.77 |
| 17 | 30 | 30 | 7 | 919.71 |
| 18 | 50 | 40 | 1.55 | 1063.53 |
| 19 | 16.36 | 40 | 15 | 900.78 |
| 20 | 30 | 50 | 7 | 990.36 |
2.2.2. Fitting the Model
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 64580.50 | 9 | 7175.61 | 30.93 | <0.0001 | significant |
| X1 (ethanol concentration) | 3526.02 | 1 | 3526.02 | 15.20 | 0.0030 | |
| X2 (ratio of solvent to material) | 20659.29 | 1 | 20659.29 | 89.04 | <0.0001 | |
| X3 (ultrasonic time) | 571.30 | 1 | 571.30 | 2.46 | 0.1477 | |
| X1X2 | 1497.85 | 1 | 1497.85 | 6.46 | 0.0293 | |
| X1X3 | 2201.69 | 1 | 2201.69 | 9.49 | 0.0116 | |
| X2X3 | 1014.57 | 1 | 1014.57 | 4.37 | 0.063 | |
| X12 | 33523.51 | 1 | 33523.51 | 144.48 | <0.0001 | |
| X22 | 3044.43 | 1 | 3044.43 | 13.12 | 0.0047 | |
| X32 | 1272.59 | 1 | 1272.59 | 5.48 | 0.0412 | |
| Residual | 2320.28 | 10 | 232.03 | |||
| Lack of Fit | 1006.46 | 5 | 201.29 | 0.77 | 0.6115 | Not significant |
| Pure Error | 1313.82 | 5 | 262.76 | |||
| Cor Total | 66900.79 | 19 | ||||
| R-Squared | 0.965 | |||||
| Adj R-Squared | 0.934 |
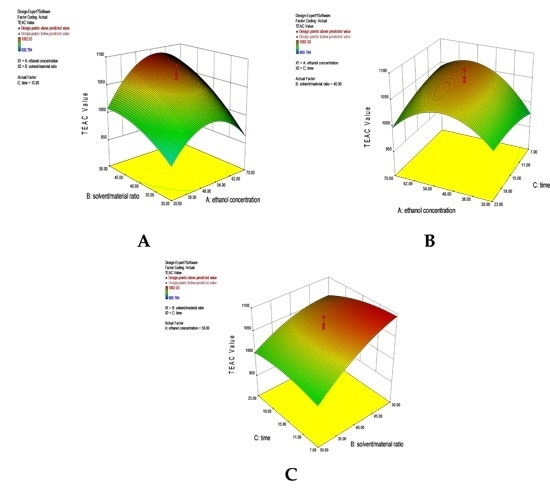
2.2.3. Analysis of Response Surfaces
2.2.4. Verification of Predicted Value of the Models
| Optimal Condition | TEAC Value (µmol Trolox/g DW) | |||
|---|---|---|---|---|
| Ethanol Concentration (%) | Solvent/Material Ratio (mL/g) | Time (min) | Experimental | Predicted |
| 59.6 | 50 | 7 | 1103.38 ± 16.11 | 1105.49 |


2.3. Comparison of Ultrasound-Assisted Extraction with Maceration and Soxhlet Extraction Methods
| Extracting Method | Ethanol Concerntration | Temperature | Time | TEAC Value (µmol Trolox/g DW) |
|---|---|---|---|---|
| UAE | 59.63% | 40 °C | 7 min | 1103.38 ± 16.11 |
| Maceration extraction | 59.63% | 25 °C | 24 h | 1022.65 ± 42.32 |
| Soxhlet extraction | 59.63% | 95 °C | 4 h | 588.06 ± 10.47 |
3. Experimental Section
3.1. Chemicals
3.2. Instruments
3.3. Sample Preparation
3.4. Extraction Procedures of Antioxidants
3.4.1. Ultrasound-assisted Extraction
3.4.2. Maceration Extraction
3.4.3. Soxhlet Extraction
3.5. Determination of Antioxidant Capacity
3.6. Experimental Design
3.6.1. Single-Factor Experiments
3.6.2. Response Surface Methodology Experiments
3.7. Statistical Analysis
| Variable | Units | Symbol | Code Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Ethanol concentration | % (v/v) | X1 | 16.36 | 30 | 50 | 70 | 83.64 |
| Solvent/material ratio | mL/g | X2 | 23.18 | 30 | 40 | 50 | 56.82 |
| Ultrasonic time | min | X3 | 1.55 | 7 | 15 | 23 | 28.45 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Aybastıer, O.; Isik, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Estaca, J.; Lopez-de-Dicastillo, C.; Hernandez-Munoz, P.; Catala, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Sujatha, M.; Dhingra, M. Rapid plant regeneration from various explants of Jatropha integerrima. Plant Cell Tissue Organ Cult. 1993, 35, 293–296. [Google Scholar] [CrossRef]
- Sujatha, M.; Sivaraj, N.; Prasad, M.S. Biochemical and histological changes during in vitro organogenesis in Jatropha integerrima. Biol. Plant. 2000, 43, 167–171. [Google Scholar] [CrossRef]
- Eshilokun, A.O.; Kasali, A.A.; Ogunwande, I.A.; Walker, T.M.; Setzer, W.N. Chemical composition and antimicrobial studies of the essential oils of Jatropha integerrima Jacq (leaf and seeds). Nat. Prod. Commun. 2007, 2, 853–855. [Google Scholar]
- Wele, A.; Baragueye, C.; Ndiaye, W.; Fall, D.; Ndoye, I.; Diop, Y.; Dubosq, Y.; Bodo, B. Cytotoxic activity of two cyclic peptides from the latex of Jatropha integerrima Euphorbiaceae. Dakar Med. 2006, 52, 209–215. [Google Scholar]
- Zhu, J.Y.; Lou, L.L.; Guo, Y.Q.; Li, W.; Guo, Y.H.; Bao, J.M.; Tang, G.H.; Bu, X.Z.; Yin, S. Natural thioredoxin reductase inhibitors from Jatropha integerrima. RSC Adv. 2015, 5, 47235–47243. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food. 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. An investigation of the mechanisms of ultrasonically enhanced desorption. AICHE J. 2007, 53, 363–373. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Majd, M.H.; Rajaei, A.; Bashi, D.S.; Mortazavi, S.A.; Bolourian, S. Optimization of ultrasonic-assisted extraction of phenolic compounds from bovine pennyroyal (Phlomidoschema parviflorum) leaves using response surface methodology. Ind. Crop. Prod. 2014, 57, 195–202. [Google Scholar] [CrossRef]
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food chem. 2014, 155, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Rosello-Soto, E.; Galanakis, C.M.; Brncic, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzerf, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Thanh, D.T.; Bhuyan, D.J.; Goldsmith, C.D.; Sadeqzadeh, E.; Scarlett, C.J.; Bowyer, M.C. Optimization of ultrasound-assisted extraction conditions for euphol from the medicinal plant, Euphorbia tirucalli, using response surface methodology. Ind. Crop. Prod. 2015, 63, 197–202. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason. Sonochem. 2008, 15, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Vankar, P.S.; Srivastava, J. Ultrasound-assisted extraction in different solvents for phytochemical study of Canna indica. Int. J. Food Eng. 2010, 6, 1556–3758. [Google Scholar] [CrossRef]
- Zou, T.B.; Xia, E.Q.; He, T.P.; Huang, M.Y.; Jia, Q.; Li, H.W. Ultrasound-assisted Extraction of Mangiferin from Mango (Mangifera indica L.) leaves using response surface methodology. Molecules 2014, 19, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A. M.; Gilca, I. A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [PubMed]
- Li, A.N.; Li, S.; Xu, D.P.; Xu, X.R.; Chen, Y.M.; Ling, W.H.; Chen, F.; Li, H.B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Meth. 2015, 8, 1207–1214. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng. 2009, 93, 166–171. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
- Oancea, S.; Grosu, C.; Ketney, O.; Stoia, M. Conventional and ultrasound-assisted extraction of anthocyanins from blackberry and sweet cherry cultivars. Acta Chim. Slov. 2013, 60, 383–389. [Google Scholar] [PubMed]
- Cui, H.Y.; Murthy, H.N.; Moh, S.H.; Cui, Y.Y.; Lee, E.J.; Paek, K.Y. Comparison of conventional and ultrasound-assisted methods for extraction of nutraceutical compounds from Dendrobium candidum. CyTA J. Food 2014, 12, 355–359. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Chemat, F.; Li, Y.; Tomao, V.; Ginies, C.; Cravotto, G. Optimization of procedures for in-line extraction of lipids and polyphenols from grape seeds. Food Anal. Meth. 2014, 7, 459–464. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of ultrasonic-assisted extraction of flavonoid compounds and antioxidants from Alfalfa using response surface method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.-P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.-N.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology. Molecules 2016, 21, 18. https://doi.org/10.3390/molecules21010018
Xu D-P, Zhou Y, Zheng J, Li S, Li A-N, Li H-B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology. Molecules. 2016; 21(1):18. https://doi.org/10.3390/molecules21010018
Chicago/Turabian StyleXu, Dong-Ping, Yue Zhou, Jie Zheng, Sha Li, An-Na Li, and Hua-Bin Li. 2016. "Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology" Molecules 21, no. 1: 18. https://doi.org/10.3390/molecules21010018