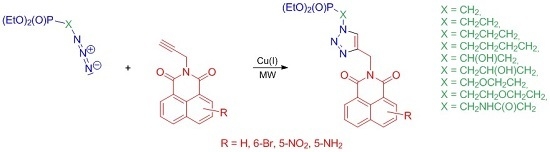
3.5. General Procedure for Copper(I)-Catalyzed Cycloaddition Reactions
To a solution of azidoalkylphosphonate 13a–i (1.00 mmol) in EtOH (1.0 mL) and H2O (1.0 mL), CuSO4 × 5H2O (0.10 mmol), sodium ascorbate (0.20 mmol) and the respective N-propargyl naphthalimides 7/8–11/12 (1.00 mmol) were added. The reaction mixture was microwave irradiated at 35–40 °C for 15 min. After removal of solvents the residue was suspended in chloroform (5 mL), filtered through a layer of Celite and concentrated in vacuo. Crude products were purified on silica gel columns with chloroform–methanol mixtures (100:1 or 50:1, v/v) or crystallized from the appropriate solvents to give the 1,2,3-triazoles 14a–i–17a–i.
Diethyl {4-[1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate (14a): Yield 81% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 110–111 °C; IR (KBr): ν = 3446, 3245, 3234, 2983, 2931, 1700, 1659, 1236, 1023, 782, cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.54 (dd, J = 7.3 Hz, J = 1.2 Hz, 2H, Haromat.), 8.16 (dd, J = 8.1 Hz, J = 1.2 Hz, 2H, Haromat.), 7.86 (s, 1H, HC5′), 7.70 (dd, J = 8.1 Hz, J = 7.3 Hz, 2H, Haromat.), 5.49 (s, 2H, CH2), 4.71 (d, J = 13.0 Hz, 2H, PCH2), 4.15–4.00 (m, 4H, 2 × POCH2CH3), 1.24 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.81, 144.18, 134.14, 131.63, 131.46, 128.24, 126.92, 124.27, 122.51, 63.46 (d, J = 6.6 Hz, POC), 45.83 (d, J = 155.3 Hz, PC), 35.18, 16.24 (d, J = 5.7 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 16.57 ppm. Anal. Calcd. for C20H21N4O5P: C, 56.08; H, 4.94; N, 13.08. Found: C, 56.21; H, 4.76; N, 12.84.
Diethyl 2-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate (14b): Yield 79% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 63–64 °C; IR (KBr): ν = 3422, 3245, 2981, 2927, 1701, 1659, 1588, 1237, 1026, 780 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.62 (dd, J = 7.3 Hz, J = 1.2 Hz, 2H, Haromat.), 8.22 (dd, J = 8.3 Hz, J = 1.2 Hz, 2H, Haromat.), 7.75 (dd, J = 8.3 Hz, J = 7.3 Hz, 2H, Haromat.), 7.69 (s, 1H, HC5′), 5.51 (s, 2H, CH2), 4.62–4.48 (m, 2H, PCH2CH2), 4.12–3.98 (m, 4H, 2 × POCH2CH3), 2.46–2.29 (m, 2H, PCH2), 1.26 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.85, 143.77, 134.78, 131.63, 131.49, 128.23, 126.95, 123.52, 122.51, 62.10 (d, J = 6.5 Hz, POC), 44.47 (PCC), 35.23, 27.29 (d, J = 143.0 Hz, PC), 16.32 (d, J = 5.9 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 26.30 ppm. Anal. Calcd. for C21H23N4O5P × 0.5H2O: C, 55.87; H, 5.36; N, 12.41. Found: C, 55.93; H, 5.15; N, 12.38.
Diethyl 3-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate (14c): Yield 78% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 131–132 °C; IR (KBr): ν = 3440, 3143, 2984, 1703, 1662, 1590, 1236, 1050, 785, 755 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.64 (d, J = 7.3 Hz, 2H, Haromat.), 8.22 (d, J = 8.3 Hz, 2H, Haromat.), 7.77 (dd, J = 8.3 Hz, J = 7.3 Hz, 2H, Haromat.), 7.69 (s, 1H, HC5′), 5.53 (s, 2H, CH2), 4.41 (t, J = 6.9 Hz, 2H, PCH2CH2CH2), 4.12–4.03 (m, 4H, 2 × POCH2CH3), 2.20 (dqu, J = 21.7 Hz, J = 6.9 Hz, 2H, PCH2CH2), 1.72 (dt, J = 18.6 Hz, J = 8.0 Hz, 2H, PCH2), 1.30 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.84, 143.52, 134.12, 131.64, 131.48, 128.25, 126.92, 123.40, 122.55, 61.74 (d, J = 6.5 Hz, POC), 50.02 (d, J = 15.5 Hz, PCCC), 35.25, 23.65 (d, J = 4.5 Hz, PCC), 22.66 (d, J = 143.5 Hz, PC), 16.39 (d, J = 6.2 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 29.99 ppm. Anal. Calcd. for C22H25N4O5P: C, 57.89; H, 5.52; N, 12.27. Found: C, 57.73; H, 5.23; N, 12.26.
Diethyl 4-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate (14d): Yield 85% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 131–132 °C; IR (KBr): ν = 3399, 3142, 3073, 2982, 2875, 1702, 1663, 1626, 1590, 1236, 1031, 786 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.61 (dd, J = 7.3 Hz, J = 1.0 Hz, 2H, Haromat.), 8.20 (d, J = 8.3 Hz, J = 1.0 Hz, 2H, Haromat.), 7.74 (dd, J = 8.3 Hz, J = 7.3 Hz, 2H, Haromat.), 7.63 (s, 1H, HC5′), 5.50 (s, 2H, CH2), 4.30 (t, J = 7.2 Hz, 2H, PCCCCH2), 4.13–3.95 (m, 4H, 2 × POCH2CH3), 2.00 (qu, J = 6.9 Hz, 2H, PCCCH2), 1.80–1.49 (m, 4H, PCCH2 and PCH2), 1.30 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.88, 143.73, 134.11, 131.63, 131.46, 128.25, 126.92, 123.13 122.56, 61.54 (d, J = 6.0 Hz, 2 × POC), 49.63, 35.27, 30.76 (d, J = 15.9 Hz, PCCC), 25.00 (d, J = 141.9 Hz, PC), 19.72 (d, J = 4.5 Hz, PCC), 16.41 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 31.78 ppm. Anal. Calcd. for C23H27N4O5P: C, 58.72; H, 5.78; N, 11.91. Found: C, 58.66; H, 5.64; N, 11.86.
Diethyl 2-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate (14e): Yield 80% (after crystallization from a methanol–diethyl ether mixture). A white solid; m.p. 186–188 °C; IR (KBr): ν = 3300, 3192, 2983, 1704, 1690, 1221, 1040, 1024, 787 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.59 (dd, J = 7.3 Hz, J = 0.8 Hz, 2H, Haromat.), 8.19 (dd, J = 8.2 Hz, J = 0.8 Hz, 2H, Haromat.), 7.85 (s, 1H, HC5′), 7.75 (dd, J = 8.2 Hz, J = 7.3 Hz, 2H, Haromat.), 5.51 (AB, J = 14.5 Hz, 1H, CHaHb), 5.49 (AB, J = 14.5 Hz, 1H, CHaHb), 4.78 (ddd, J = 14.3 Hz, J = 6.2 Hz, J = 2.5 Hz, 1H, PCCHaHb), 4.38 (ddd, J = 14.3 Hz, J = 9.6 Hz, J = 5.5 Hz, 1H, PCCHaHb), 4.35 (dt, J = 9.6 Hz, J = 2.5 Hz, 1H, PCH), 4.22–4.11 (m, 4H, 2 × POCH2CH3), 1.35 (t, J = 7.0 Hz, 3H, POCH2CH3), 1.32 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.74, 143.38, 134.04, 131.50, 131.35, 128.07, 126.87, 124.98, 122.2, 67.19 (d, J = 164.6 Hz, PC), 63.58 and 63.24 (2 × d, J = 7.6 Hz, 2 × POC), 51.57 (d, J = 9.1 Hz, PCC), 35.20, 16.42 and 16.38 (2 × d, J = 6.0 Hz, 2 × POCC); 31P-NMR (243 MHz, CDCl3): δ = 19.89 ppm. Anal. Calcd. for C21H23N4O6P: C, 55.02; H, 5.06; N, 12.22. Found: C, 55.10; H, 4.88; N, 12.22.
Diethyl 3-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate (14f): Yield 90% (after crystallization from a methanol–diethyl ether mixture). A white solid; m.p. >260 °C; IR (KBr): ν = 3430, 3148, 2986, 2930, 1776, 1740, 1237, 1029, 755 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.60 (dd, J = 7.9 Hz, J = 0.9 Hz, 2H, Haromat.), 8.20 (dd, J = 8.3 Hz, J = 0.9 Hz, 2H, Haromat.), 7.82 (s, 1H, HC5′), 7.73 (dd, J = 7.9 Hz, J = 8.3 Hz, 2H, Haromat.), 5.51 (s, 2H, CH2), 4.55–4.27 (m, 3H, PCCCH2, OH), 4.19–3.96 (m, 5H, PCCH, 2 × POCH2CH3), 2.06–1.67 (m, 2H, PCH2), 1.32 and 1.26 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.83, 143.63, 134.07, 131.59, 131.44, 128.21, 126.90, 124.85, 122.53, 65.62 (d, J = 3.0 Hz, PCC), 62.21 (d, J = 6.4 Hz, POC), 62.18 (d, J = 6.4 Hz, POC), 55.72 (d, J = 16.6 Hz, PCCC), 35.26, 30.59 (d, J = 139.8 Hz, PC), 16.30 (d, J = 6.0 Hz, 2 × POCC); 31P-NMR (81 MHz, CDCl3): δ = 29.28 ppm. Anal. Calcd. for C22H25N4O6P: C, 55.93; H, 5.33; N, 11.86. Found: C, 55.79; H, 5.14; N, 11.86.
Diethyl 2-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy-methylphosphonate (14g): Yield 82% (after crystallization from a methanol–diethyl ether mixture). A white solid; m.p. 154–155 °C; IR (KBr): ν = 3319, 3148, 3068, 3988, 2980, 2908, 1704, 1662, 1590, 1237, 1029, 784, 756 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.60 (dd, J = 7.3 Hz, J = 1.0 Hz, 2H, Haromat.), 8.19 (dd, J = 8.3 Hz, J = 1.0 Hz, 2H, Haromat.), 7.77 (s, 1H, HC5′), 7.74 (dd, J = 8.3 Hz, J = 7.3 Hz, 2H, Haromat.), 5.50 (s, 2H, CH2), 4.50 (t, J = 4.9 Hz, 2H, PCH2OCH2CH2), 4.15–4.00 (m, 4H, 2 × POCH2CH3) 3.95 (t, J = 4.9 Hz, 2H, PCOCH2CH2), 3.74 (d, J = 8.2 Hz, 2H, PCH2O), 1.28 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.82, 143.76, 134.09, 131.63, 131.43, 128.24, 126.92, 123.90, 122.57, 71.35 (d, J = 10.1 Hz, PCOC), 65.36 (d, J = 166.3 Hz, PC), 62.40 (d, J = 6.5 Hz, POC), 50.02, 35.25, 16.42 (d, J = 6.0 Hz, 2 × POCC); 31P-NMR (81 MHz, CDCl3): δ = 21.16 ppm. Anal. Calcd. for C22H25N4O6P: C, 55.93; H, 5.33; N, 11.86. Found: C, 55.90; H, 5.28; N, 11.84.
Diethyl 2-(2-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy)ethylphosphonate (14h): Yield 79% (after crystallization from a methanol–diethyl ether mixture). A white solid; m.p. 156–158 °C; IR (KBr): ν = 3352, 3144, 2985, 2932, 2906, 1703, 1662, 1237, 1028, 785, 754 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.60 (dd, J = 7.2 Hz, J = 0.9 Hz, 2H, Haromat.), 8.22 (dd, J = 7.9 Hz, J = 0.9 Hz, 2H, Haromat.), 7.78 (s, 1H, HC5′), 7.74 (dd, J = 7.9 Hz, J = 7.2 Hz, 1H, Haromat.), 5.59 (s, 2H, CH2), 4.47 (t, J = 5.3 Hz, 2H, PCH2CH2OCH2CH2), 4.11–3.97 (m, 4H, 2 × POCH2CH3), 3.77 (t, J = 5.3 Hz, 2H, PCCOCH2CH2), 3.64 (dt, J = 11.8 Hz, J = 7.6 Hz, 2H, PCH2CH2O), 2.03 (dt, J = 18.7 Hz, J = 7.6 Hz, 2H, PCH2CH2O), 1.28 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.86, 143.56, 134.07, 131.63, 131.44, 128.25, 126.92, 124.28, 122.60, 68.90, 65.23 (PCCO), 61.65 (d, J = 6.0 Hz, POC), 50.06, 35.27, 26.33 (d, J = 138.9 Hz, PC), 16.39 (d, J = 6.1 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 28.75 ppm. Anal. Calcd. for C23H27N4O6P: C, 56.79; H, 5.59; N, 11.52. Found: C, 56.72; H, 5.42; N, 11.70.
Diethyl 2-{4-[(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}acetamido-methylphosphonate (14i): Yield 91% (after column chromatography with chloroform–methanol mixtures (100:1 or 50:1, v/v)). A white powder; m.p. 170–171 °C; IR (KBr): ν = 3355, 2974, 2930, 1660, 1626, 1237, 1050, 782 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.51 (dd, J = 7.3 Hz, J = 1.1 Hz, 2H, Haromat.), 8.12 (dd, J = 8.2 Hz, J = 1.1 Hz, 2H, Haromat.), 7.80 (s, 1H, HC5′), 7.66 (dd, J = 8.2 Hz, J = 7.3 Hz, 2H, Haromat.), 7.49 (t, J = 5.9 Hz, 1H, NHCO), 5.44 (s, 2H, CH2), 5.01 (s, 2H), 4.06–3.92 (m, 4H, 2 × POCH2CH3), 3.62 (dd, J = 12.2 Hz, J = 5.9 Hz, 2H, PCH2NH), 1.17 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 165.24 (d, J = 5.5 Hz, C=O), 163.82, 144.09, 134.15, 131.60, 131.48, 128.20, 126.93, 124.93, 122.47, 62.84 (d, J = 6.5 Hz, POC), 52.56, 35.17, 34.97 (d, J = 156.5 Hz, PC), 16.28 (d, J = 5.6 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 22.21 ppm. Anal. Calcd. for C22H24N5O6P: C, 54.43; H, 4.98; N, 14.43. Found: C, 54.38; H, 4.82; N, 14.35.
Diethyl {4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate (15a): Yield 82% (after column chromatography with chloroform–methanol mixtures (100:1 and 50:1, v/v)). A white powder; m.p. 158–159 °C; IR (KBr): ν = 3334, 3052, 3008, 2989, 2967, 1711, 1670, 1222, 1032, 757 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.67 (d, J = 7.8 Hz, 1H, Haromat.), 8.59 (d, J = 8.5 Hz, 1H, Haromat.), 8.46 (d, J = 7.8 Hz, 1H, Haromat.), 8.05 (d, J = 7.8 Hz, 1H, Haromat.), 7.88 (s, 1H, HC5′), 7.86 (dd, J = 8.5 Hz, J = 7.8 Hz, 1H, Haromat.), 5.53 (s, 2H, CH2), 4.74 (d, J = 13.0 Hz, 2H, PCH2), 4.15–4.00 (m, 4H, 2 × POCH2CH3), 1.29 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.24, 163.21, 143.87, 133.49, 132.28, 131.46, 131.13, 130.68, 130.52, 129.07, 128.08, 124.30, 122.94, 122.07, 63.45 (d, J = 6.4 Hz, POC), 45.63 (d, J = 156.4 Hz, PC), 35.26, 16.25 (d, J = 6.8 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 16.65 ppm. Anal. Calcd. for C20H20BrN4O5P: C, 47.35; H, 3.97; N, 11.04. Found: C, 47.31; H, 3.73; N, 10.99.
Diethyl 2-{4-(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl)-1H-1,2,3-triazol-1-yl}ethylphosphonate (15b): Yield 80% (after crystallization from ethyl acetate). A white solid; m.p. 132–134 °C; IR (KBr): ν = 3400, 3352, 3308, 2985, 2932, 1703, 1666, 1232, 1026, 779, 750 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.61 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.50 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.37 (d, J = 8.3 Hz, 1H, Haromat.), 7.98 (d, J = 7.9 Hz, 1H, Haromat.), 7.78 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.64 (s, 1H, HC5′), 5.48 (s, 2H, CH2), 4.57–4.43 (m, 2H, PCH2CH2), 4.07–3.93 (m, 4H, 2 × POCH2CH3), 2.41–2.29 (m, 2H, PCH2), 1.21 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.15, 163.14, 143.43, 133.42, 132.22, 131.38, 131.09, 130.58, 130.48, 128.94, 128.06, 123.58, 122.85, 121.98, 62.09 (d, J = 6.5 Hz, POC), 44.48 (PCC), 35.28, 27.26 (d, J = 141.0 Hz, PC), 16.32 (d, J = 5.8 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 26.28 ppm. Anal. Calcd. for C21H22BrN4O5P: C, 48.38; H, 4.25; N, 10.75. Found: C, 48.07; H, 4.10; N, 10.62.
Diethyl 3-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate (15c): Yield 76% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 118–120 °C; IR (KBr): ν = 3404, 2990, 2942, 2829, 1705, 1666, 1590, 1234, 1047, 1029, 752 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.68 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.56 (dd, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.42 (d, J = 7.9 Hz, 1H, Haromat.), 8.04 (d, J = 7.9 Hz, 1H, Haromat.), 7.84 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.67 (s, 1H, HC5′), 5.49 (s, 2H, CH2), 4.40 (t, J = 7.0 Hz, 2H, PCH2CH2CH2), 4.15–3.96 (m, 4H, 2 × POCH2CH3), 2.18 (dqu, J = 21.0 Hz, J = 7.0 Hz, 2H, PCH2CH2), 1.72 (dt, J = 18.9 Hz, J = 8.0 Hz, 2H, PCH2), 1.28 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.29, 163.26, 143.50, 133.49, 132.31, 131.47, 131.12, 130.68, 130.51, 129.09, 128.08, 123.48, 122.97, 122.10, 61.76 (d, J = 6.5 Hz, POC), 50.02 (d, J = 15.1 Hz, PCCC), 35.32, 23.64 (d, J = 4.5 Hz, PCC), 22.66 (d, J = 143.5 Hz, PC), 16.40 (d, J = 5.7 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 30.85 ppm. Anal. Calcd. for C22H24BrN4O5P: C, 49.36; H, 4.52; N, 10.47. Found: C, 49.25; H, 4.44; N, 10.45.
Diethyl 4-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate (15d): Yield 88% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 110–112 °C; IR (KBr): ν = 3357, 2982, 2938, 2909, 2875, 1794, 1703, 1024, 962 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.67 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.57 (d, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.42 (d, J = 7.9 Hz, 1H, Haromat.), 8.04 (d, J = 7.9 Hz, 1H, Haromat.), 7.84 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.63 (s, 1H, HC5′), 5.48 (s, 2H, CH2), 4.31 (t, J = 7.2 Hz, 2H, PCCCCH2), 4.12–3.97 (m, 4H, 2 × POCH2CH3), 2.12–1.90 (m, 2H, PCCCH2), 1.84–1.49 (m, 4H, PCCH2 and PCH2), 1.30 (t, J = 7.2 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.25, 163.22, 143.41, 133.43, 132.26, 131.43, 131.09, 130.65, 130.50, 129.05, 128.06, 123.17, 122.96, 122.08, 61.54 (d, J = 7.6 Hz, 2 × POC), 49.64, 35.33, 30.74 (d, J = 13.6 Hz, PCCC), 24.95 (d, J = 143.5 Hz, PC), 19.71 (d, J = 4.5 Hz, PCC), 16.40 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 31.77 ppm. Anal. Calcd. for C23H26BrN4O5P: C, 50.29; H, 4.77; N, 10.20. Found: C, 50.11; H, 4.62; N, 10.00.
Diethyl 2-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate (15e): Yield 80% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 198–200 °C; IR (KBr): ν = 3261, 2987, 2933, 2909, 1704, 1665, 1234, 1046, 1023, 753 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.50 (dd, J = 7.3 Hz, J = 1.1 Hz, 1H, Haromat.), 8.44 (d, J = 8.3 Hz, J = 1.1 Hz, 1H, Haromat.), 8.26 (d, J = 7.9 Hz, 1H, Haromat.), 7.94 (d, J = 7.9 Hz, 1H, Haromat.), 7.88 (s, 1H, HC5′), 7.75 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 5.41 (s, 2H, CH2), 4.79 (ddd, J = 11.3 Hz, J = 6.3 Hz, J = 2.3 Hz, 1H, PCCHaHb), 4.46 (dt, J = 9.9 Hz, J = 2.3 Hz, 1H, PCH), 4.38 (ddd, J = 11.3 Hz, J = 9.9 Hz, J = 5.0 Hz, 1H, PCCHaHb), 4.21–4.06 (m, 4H, 2 × POCH2CH3), 1.31 (t, J = 6.9 Hz, 3H, POCH2CH3), 1.29 (t, J = 6.9 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.09, 163.00, 143.05, 133.36, 132.15, 131.31, 131.07, 130.45, 128.78, 128.02, 126.87, 125.10, 122.73, 121.87, 67.20 (d, J = 164.6 Hz, PC), 63.40 (d, J = 7.6 Hz, POC), 63.25 (d, J = 7.6 Hz, POC), 51.64 (d, J = 9.1 Hz, PCC), 35.27, 16.44 (d, J = 6.0 Hz, POCC), 16.40 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 20.91 ppm. Anal. Calcd. for C21H22BrN4O6P: C, 46.94; H, 4.13; N, 10.43. Found: C, 47.07; H, 3.91; N, 10.48.
Diethyl 3-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate (15f): Yield 85% (after crystallization from a methanol–diethyl ether mixture). A white solid; m.p. 152–153 °C; IR (KBr): ν = 3330, 3155, 2986, 2909, 1704, 1664, 1234, 1027, 752 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.54 (dd, J = 7.5 Hz, J = 1.0 Hz, 1H, Haromat.), 8.48 (d, J = 8.5 Hz, J = 1.0 Hz, 1H, Haromat.), 8.29 (d, J = 7.9 Hz, 1H, Haromat.), 7.96 (d, J = 7.9 Hz, 1H, Haromat.), 7.90 (s, 1H, HC5′), 7.65 (dd, J = 8.5 Hz, J = 7.5 Hz, 1H, Haromat.), 5.49 (s, 2H, CH2), 4.52–4.17 (m, 3H, PCCCH2, OH), 4.16–3.95 (m, 5H, PCCH, 2 × POCH2CH3), 2.10–1.60 (m, 2H, PCH2), 1.33 and 1.29 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.30, 163.28, 143.33, 133.47, 132.31, 131.47, 131.12, 130.64, 130.50, 129.05, 128.07, 124.95, 122.94, 122.07, 65.61 (d, J = 4.4 Hz, PCC), 62.30 (d, J = 6.0 Hz, POC), 62.20 (d, J = 6.0 Hz, POC), 55.75 (d, J = 17.6 Hz, PCCC), 35.34, 30.59 (d, J = 141.8 Hz, PC), 16.30 (d, J = 6.0 Hz, 2 × POCC); 31P-NMR (81 MHz, CDCl3): δ = 29.21 ppm. Anal. Calcd. for C22H24BrN4O6P: C, 47.93; H, 4.39; N, 10.16. Found: C, 47.94; H, 4.50; N, 10.16.
Diethyl 2-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy-methylphosphonate (15g): Yield 90% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 127–128 °C; IR (KBr): ν = 3441, 3148, 3087, 2985, 2935, 2908, 1704, 1665, 1234, 1027, 751 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.51 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.47 (d, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.45 (d, J = 7.9 Hz, 1H, Haromat.), 7.99 (d, J = 7.9 Hz, 1H, Haromat.), 7.74 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.70 (s, 1H, HC5′), 5.49 (s, 2H, CH2), 4.49 (t, J = 5.1 Hz, 2H, PCH2OCH2CH2), 4.18–3.98 (m, 4H, 2 × POCH2CH3), 3.94 (t, J = 5.1 Hz, 2H, PCOCH2CH2), 3.70 (d, J = 8.1 Hz, 2H, PCH2O), 1.30 (t, J = 7.2 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.25, 163.23, 143.32, 133.44, 132.26, 131.43, 131.19, 130.67, 130.45, 129.08, 128.08, 124.21, 123.00, 122.13, 71.32 (d, J = 9.1 Hz, PCOC), 65.16 (d, J = 166.1 Hz, PC), 62.47 (d, J = 6.3 Hz, POC), 50.03, 35.32, 16.44 (d, J = 5.4 Hz, 2 × POCC); 31P-NMR (81 MHz, CDCl3): δ = 21.17 ppm. Anal. Calcd. for C22H24BrN4O6P: C, 47.93; H, 4.39; N, 10.16. Found: C, 48.07; H, 4.10; N, 9.97.
Diethyl 2-(2-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy)ethylphosphonate (15h): Yield 86% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 92–93 °C; IR (KBr): ν = 3145, 3086, 2984, 2930, 2907, 2876, 1704, 1666, 1234, 1047, 751 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.72 (dd, J = 7.3 Hz, J = 1.0 Hz, 1H, Haromat.), 8.55 (d, J = 8.3 Hz, J = 1.0 Hz, 1H, Haromat.), 8.49 (d, J = 7.9 Hz, 1H, Haromat.), 8.10 (d, J = 7.9 Hz, 1H, Haromat.), 7.84 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.76 (s, 1H, HC5′), 5.52 (s, 2H, CH2), 4.49 (t, J = 5.3 Hz, 2H, PCH2CH2OCH2CH2), 4.18–3.95 (m, 4H, 2 × POCH2CH3), 3.79 (t, J = 5.3 Hz, 2H, PCCOCH2CH2), 3.56 (dt, J = 12.0 Hz, J = 7.5 Hz, 2H, PCH2CH2O), 2.03 (dt, J = 18.4 Hz, J = 7.5 Hz, 2H, PCH2CH2O), 1.25 (t, J = 6.9 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 163.27, 163.25, 143.28, 133.40, 132.26, 131.43, 131.11, 130.66, 130.42, 129.09, 128.07, 124.31, 123.03, 122.16, 68.97, 65.24 (PCCO), 61.63 (d, J = 5.7 Hz, POC), 50.08, 35.36, 26.10 (d, J = 140.1 Hz, PC), 16.40 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 28.76 ppm. Anal. Calcd. for C23H26BrN4O6P: C, 48.86; H, 4.64; N, 9.91. Found: C, 48.83; H, 4.56; N, 10.10.
Diethyl 2-{4-[(6-Bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}acetamido-methylphosphonate (15i): Yield 88% (after column chromatography with chloroform–methanol mixtures (100:1 or 50:1, v/v)). A white solid; m.p. 173–174 °C; IR (KBr): ν = 3240, 3148, 3071, 2987, 2932, 1703, 1665, 1234, 1025, 752 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.52 (dd, J = 7.3 Hz, J = 0.8 Hz, 1H, Haromat.), 8.42 (d, J = 8.3 Hz, J = 0.8 Hz, 1H, Haromat.), 8.39 (d, J = 7.9 Hz, 1H, Haromat.), 8.00 (d, J = 7.9 Hz, 1H, Haromat.), 7.84 (s, 1H, HC5′), 7.80 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.40 (brt, J = 5.9 Hz, 1H, NHCO), 5.56 (s, 2H, CH2), 5.01 (s, 2H), 4.16–3.98 (m, 4H, 2 × POCH2CH3), 3.62 (dd, J = 12.4 Hz, J = 6.0 Hz, 2H, PCH2NH), 1.12 (t, J = 7.2 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 165.26 (d, J = 5.5 Hz, C=O), 163.25, 163.24, 143.76, 133.49, 132.29, 131.45, 131.12, 130.62, 130.54, 128.99, 128.07, 125.02, 122.85, 121.98, 62.87 (d, J = 6.6 Hz, POC), 52.57, 35.25, 35.00 (d, J = 155.4 Hz, PC), 16.30 (d, J = 5.4 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 22.29 ppm. Anal. Calcd. for C22H23BrN5O6P: C, 46.82; H, 4.11; N, 12.41. Found: C, 46.62; H, 3.90; N, 12.11.
Diethyl {4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate (16a): Yield 71% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 147–148 °C; IR (KBr): ν = 3335, 2988, 2939, 1698, 1711, 1670, 1244, 1110, 790, 757 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.24 (d, J = 2.2 Hz, 1H, Haromat.), 9.07 (d, J = 2.2 Hz, 1H, Haromat.), 8.73 (dd, J = 7.4 Hz, J = 1.2 Hz, 1H, Haromat.), 8.36 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, Haromat.), 7.88 (dd, J = 8.4 Hz, J = 7.4 Hz, 1H, Haromat.), 7.84 ( s, 1H, HC5′), 5.47 (s, 2H, CH2), 4.66 (d, J = 13.1 Hz, 2H, PCH2), 4.13–3.98 (m, 4H, 2 × POCH2CH3), 1.22 (2 × t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.70, 162.17, 146.36, 143.43, 135.78, 134.68, 131.05, 130.23, 129.12, 129.11, 124.52, 124.39, 124.35, 123.04, 63.50 (d, J = 6.6 Hz, POC), 45.36 (d, J = 155.6 Hz, PC), 35.47, 16.27 (d, J = 5.6 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 16.50 ppm. Anal. Calcd. for C20H20N5O7P: C, 50.74; H, 4.26; N, 14.79. Found: C, 50.73; H, 3.99; N, 14.93.
Diethyl 2-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate (16b): Yield 88% (after crystallization from an ethyl acetate–hexane mixture). White needles; m.p. 170–171 °C; IR (KBr): ν = 3284, 3142, 3079, 2934, 2910, 1713, 1667, 1244, 790, 703 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.26 (d, J = 2.2 Hz, 1H, Haromat.), 9.08 (d, J = 2.2 Hz, 1H, Haromat.), 8.74 (dd, J = 7.3 Hz, J = 1.1 Hz, 1H, Haromat.), 8.38 (dd, J = 8.3 Hz, J = 1.1 Hz, 1H, Haromat.), 7.89 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.68 (s, 1H, HC5′), 5.46 (s, 2H, CH2), 4.58–4.45 (m, 2H, PCH2CH2), 4.09–3.94 (m, 4H, 2 × POCH2CH3), 2.42‒2.24 (m, 2H, PCH2), 1.23 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.74, 162.23, 146.40, 143.04, 135.77, 134.72, 131.06, 130.25, 129.13, 129.13, 124.54, 124.46, 123.61, 123.07, 62.14 (d, J = 6.0 Hz, POC), 44.55 (PCC), 35.50, 27.30 (d, J = 140.4 Hz, PC), 16.34 (d, J = 5.7 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 26.23 ppm. Anal. Calcd. for C21H22N4O7P: C, 51.75; H, 4.55; N, 14.37. Found: C, 51.54; H, 4.43; N, 14.17.
Diethyl 3-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate (16c): Yield 80% (after crystallization from ethyl acetate). A white solid; m.p. 113–114 °C; IR (KBr): ν = 3404, 3084, 2982, 2943, 1712, 1671, 1597, 1244, 1112, 1029, 791, 758 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.32 (d, J = 2.1 Hz, 1H, Haromat.), 9.13 (d, J = 2.1 Hz, 1H, Haromat.), 8.80 (dd, J = 7.3 Hz, J = 1.1 Hz, 1H, Haromat.), 8.43 (dd, J = 8.3 Hz, J = 1.1 Hz, 1H, Haromat.), 7.94 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.71 (s, 1H, HC5′), 5.52 (s, 2H, CH2), 4.41 (t, J = 7.0 Hz, 2H, PCH2CH2CH2), 4.15–3.97 (m, 4H, 2 × POCH2CH3), 2.30–2.08 (m, 2H, PCH2CH2), ), 1.80–1.60 (m, 2H, PCH2), 1.29 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.76, 162.25, 146.37, 143.06, 135.77, 134.74, 131.07, 130.26, 129.11, 129.11, 124.56, 124.43, 123.39, 123.08, 61.79 (d, J = 6.0 Hz, POC), 50.00 (d, J = 15.1 Hz, PCCC), 35.50, 23.64 (d, J = 4.4 Hz, PCC), 22.46 (d, J = 143.5 Hz, PC), 16.40 (d, J = 5.4 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 30.84 ppm. Anal. Calcd. for C22H24N5O7P: C, 52.70; H, 4.82; N, 13.97. Found: C, 52.75; H, 4.93; N, 14.01.
Diethyl 4-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate (16d): Yield 82% (after crystallization from ethyl an acetate–hexane mixture). White needles; m.p. 150–151 °C; IR (KBr): ν = 3369, 3145, 3082, 2987, 1711, 1670, 1243, 1027, 791, 754 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.31 (d, J = 2.1 Hz, 1H, Haromat.), 9.13 (d, J = 2.1 Hz, 1H, Haromat.), 8.79 (dd, J = 7.4 Hz, J = 1.1 Hz, 1H, Haromat.), 8.42 (dd, J = 8.3 Hz, J = 1.1 Hz, 1H, Haromat.), 7.94 (dd, J = 8.3 Hz, J = 7.4 Hz, 1H, Haromat.), 7.67 (s, 1H, HC5′), 5.52 (s, 2H, CH2), 4.30 (t, J = 7.1 Hz, 2H, PCCCCH2), 4.13–3.95 (m, 4H, 2 × POCH2CH3), 2.07–1.92 (m, 2H, PCCCH2), 1.82–1.53 (m, 4H, PCCH2 and PCH2), 1.28 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.75, 162.25, 146.38, 142.99, 135.73, 134.72, 131.06, 130.27, 129.11, 129.09, 124.59, 124.44, 123.28, 123.11, 61.59 (d, J = 6.6 Hz, 2 × POC), 49.71, 35.52, 30.76 (d, J = 15.8 Hz, PCCC), 25.00 (d, J = 141.9 Hz, PC), 19.72 (d, J = 4.9 Hz, PCC), 16.41 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 31.73 ppm. Anal. Calcd. for C23H26N5O7P: C, 53.59; H, 5.08; N, 13.59. Found: C, 53.56; H, 4.92; N, 13.55.
Diethyl 2-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate (16e): Yield 83% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 212–213 °C; IR (KBr): ν = 3302, 3130, 2973, 1710, 1694, 1228, 1027, 789 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.30 (d, J = 2.2 Hz, 1H, Haromat.), 9.13 (d, J = 2.2 Hz, 1H, Haromat.), 8.79 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.42 (dd, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 7.94 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.85 (s, 1H, HC5′), 5.52 (AB, J = 14.2 Hz, 1H, CHaHb), 5.49 (AB, J = 14.2 Hz, 1H, CHaHb), 4.76 (ddd, J = 13.8 Hz, J = 6.8 Hz, J = 2.6 Hz, 1H, PCCHaHb), 4.52–4.27 (m, 2H, PCCHaHb, PCH), 4.26–4.08 (m, 4H, 2 × POCH2CH3), 1.34 (t, J = 7.0 Hz, 3H, POCH2CH3), 1.32 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.74, 162.21, 146.38, 142.78, 135.73, 134.70, 131.04, 130.23, 129.12, 129.08, 124.98, 124.55, 124.41, 123.08, 67.15 (d, J = 164.6 Hz, PC), 63.46 (d, J = 6.6 Hz, POC), 63.24 (d, J = 6.6 Hz, POC), 51.55 (d, J = 9.1 Hz, PCC), 35.51, 16.44 (d, J = 6.0 Hz, POCC), 16.40 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 20.63 ppm. Anal. Calcd. for C21H22N5O8P: C, 50.10; H, 4.40; N, 13.91. Found: C, 55.02; H, 4.14; N, 13.86.
Diethyl 3-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate (16f): Yield 86% (after crystallization from an ethyl acetate–hexane mixture). A white solid; m.p. 176–177 °C; IR (KBr): ν = 3279, 3135, 3080, 2986, 2931, 2830, 1709, 1667, 1232, 1033, 799, 755 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.31 (d, J = 2.2 Hz, 1H, Haromat.), 9.13 (d, J = 2.2 Hz, 1H, Haromat.), 8.79 (dd, J = 7.4 Hz, J = 1.2 Hz, 1H, Haromat.), 8.43 (dd, J = 8.2 Hz, J = 1.2 Hz, 1H, Haromat.), 7.94 (dd, J = 8.2 Hz, J = 7.4 Hz, 1H, Haromat.), 7.86 (s, 1H, HC5′), 5.53 (s, 2H, CH2), 4.56–4.31 (m, 3H, PCCCH2, OH), 4.18–4.00 (m, 5H, PCCH, 2 × POCH2CH3), 2.06–1.74 (m, 2H, PCH2), 1.31 and 1.30 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.75, 162.22, 146.39, 142.92, 135.70, 134.70, 131.06, 130.27, 129.09, 129.06, 124.96, 124.60, 124.44, 123.12, 65.59 (d, J = 3.0 Hz, PCC), 62.32 (d, J = 6.4 Hz, POC), 62.26 (d, J = 6.4 Hz, POC), 55.75 (d, J = 16.6 Hz, PCCC), 35.55, 30.56 (d, J = 140.4 Hz, PC), 16.36 (d, J = 6.0 Hz, POCC), 16.32 (d, J = 6.0 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 29.28 ppm. Anal. Calcd. for C22H24N5O8P: C, 51.07; H, 4.68; N, 13.53. Found: C, 51.18; H, 4.43; N, 13.77.
Diethyl 2-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy-methylphosphonate (16g): Yield 82% (after crystallization from an ethyl acetate–hexane mixture). A yellow powder; m.p. 89–90 °C; IR (KBr): ν = 3397, 3019, 1712, 1672, 1215, 1048, 757 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.30 (d, J = 2.2 Hz, 1H, Haromat.), 9.12 (d, J = 2.2 Hz, 1H, Haromat.), 8.78 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.44 (dd, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 7.94 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.82 (s, 1H, HC5′), 5.51 (s, 2H, CH2), 4.52 (t, J = 4.8 Hz, 2H, PCH2OCH2CH2), 4.18–4.04 (m, 4H, 2 × POCH2CH3) 3.95 (t, J = 4.8 Hz, 2H, PCOCH2CH2), 3.75 (d, J = 8.2 Hz, 2H, PCH2O), 1.31 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.72, 162.21, 146.36, 142.92, 135.72, 134.67, 131.04, 130.26, 129.10, 129.07, 124.60, 124.37, 124.31, 123.11, 71.32 (d, J = 10.6 Hz, PCOC), 65.34 (d, J = 166.1 Hz, PC), 62.50 (d, J = 6.5 Hz, POC), 50.04, 35.52, 16.46 (d, J = 6.0 Hz, 2 × POCC); 31P-NMR (81 MHz, CDCl3): δ = 21.15 ppm. Anal. Calcd. for C22H24N5O8P: C, 51.07; H, 4.68; N, 13.53. Found: C, 51.10; H, 4.39; N, 13.62.
Diethyl 2-(2-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy)ethylphosphonate (16h): Yield 89%; (after column chromatography with chloroform–methanol mixtures (100:1 or 50:1, v/v)). A yellow oil; IR (film): ν = 3363, 3018, 2992, 1711, 1670, 1216, 1053, 755 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 9.30 (d, J = 2.2 Hz, 1H, Haromat.), 9.12 (d, J = 2.2 Hz, 1H, Haromat.), 8.78 (dd, J = 7.3 Hz, J = 1.2 Hz, 1H, Haromat.), 8.42 (dd, J = 8.3 Hz, J = 1.2 Hz, 1H, Haromat.), 7.93 (dd, J = 8.3 Hz, J = 7.3 Hz, 1H, Haromat.), 7.82 (s, 1H, HC5′), 5.52 (s, 2H, CH2), 4.48 (t, J = 5.3 Hz, 2H, PCH2CH2OCH2CH2), 4.12–3.98 (m, 4H, 2 × POCH2CH3), 3.78 (t, J = 5.3 Hz, 2H, PCCOCH2CH2), 3.66 (dt, J = 12.1 Hz, J = 7.6 Hz, 2H, PCH2CH2O), 2.05 (dt, J = 18.9 Hz, J = 7.6 Hz, 2H, PCH2CH2O), 1.29 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 162.76, 162.25, 146.40, 142.86, 135.67, 134.68, 131.06, 130.29, 129.10, 129.03, 124.65, 124.40, 124.37, 123.17, 68.96, 65.25 (PCCO), 61.68 (d, J = 6.0 Hz, POC), 50.13, 35.57, 26.87 (d, J = 140.4 Hz, PC), 16.41 (d, J = 6.1 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 28.70 ppm. Anal. Calcd. for C23H26N5O8P: C, 51.98; H, 4.93; N, 13.18. Found: C, 51.70; H, 4.67; N, 13.15.
Diethyl 2-{4-[(5-Nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}acetamido-methylphosphonate (16i): Yield 91%. A white powder; m.p. 217–219 °C; IR (KBr): ν = 3287, 3075, 2986, 2854, 1709, 1687, 1229, 1031, 758 cm−1; 1H-NMR (200 MHz, DMSO-d6): δ = 9.51 (d, J = 2.2 Hz, 1H, Haromat.), 9.00 (d, J = 2.2 Hz, 1H, Haromat.), 8.82 (dd, J = 7.5 Hz, J = 0.6 Hz, 1H, Haromat.), 8.72 (dd, J = 8.2 Hz, J = 0.6 Hz, 1H, Haromat.), 8.70 (brt, J = 2.8 Hz, 1H, NH), 8.09 (dd, J = 8.2 Hz, J = 7.5 Hz, 1H, Haromat.), 8.03 (s, 1H, HC5′), 5.36 (s, 2H, CH2), 5.10 (s, 2H), 4.19–3.97 (m, 4H, 2 × POCH2CH3), 3.59 (dd, J = 11.8 Hz, J = 6.0 Hz, 2H, PCH2NH), 1.19 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 165.93 (d, J = 5.2 Hz, C=O), 162.97, 162.51, 146.36, 142.85, 137.00, 134.60, 131.39, 130.37, 130.04, 129.76, 125.41, 124.35, 123.57, 122.91, 62.30 (d, J = 6.5 Hz, POC), 51.87, 35.97, 34.65 (d, J = 155.5 Hz, PC), 16.65 (d, J = 5.6 Hz, POCC); 31P-NMR (81 MHz, DMSO-d6): δ = 22.27 ppm. Anal. Calcd. for C22H23N6O8P: C, 49.82; H, 4.37; N, 15.84. Found: C, 49.88; H, 4.07; N, 15.64.
Diethyl {4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate (17a): Yield 75% (after column chromatography with chloroform–methanol mixtures (100:1 and 50:1, v/v)). A yellow oil; IR (film): ν = 3430, 3352, 3234, 2983, 2932, 1698, 1660, 1623, 1236, 1020, 784, 748 cm−1; 1H-NMR (600 MHz, DMSO-d6): δ = 8.10 (d, J = 7.2 Hz, 1H, Haromat.), 8.07 (d, J = 8.2 Hz, 1H, Haromat.), 8.00 (d, J = 2.2 Hz, 1H, Haromat.), 7.95 ( s, 1H, HC5′), 7.64 (dd, J = 8.2 Hz, J = 7.2 Hz, 1H, Haromat.), 7.32 (d, J = 2.2 Hz, 1H, Haromat.), 6.01 (s, 2H, NH2), 5.30 (s, 2H, CH2), 5.01 (d, J = 12.9 Hz, 2H, PCH2), 4.05–3.98 (m, 4H, 2 × POCH2CH3), 1.19 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 164.00, 163.82, 148.41, 143.74, 134.10, 132.23, 127.46, 126.08, 124.85, 122.91, 122.35, 122.11, 121.11, 112.45, 63.02 (d, J = 6.3 Hz, POC), 45.10 (d, J = 150.3 Hz, PC), 35.51, 16.47 (d, J = 5.8 Hz, POCC); 31P-NMR (243 MHz, DMSO-d6): δ = 17.22 ppm. Anal. Calcd. for C20H22N5O5P: C, 54.18; H, 5.00; N, 15.80. Found: C, 54.36; H, 4.83; N, 15.84.
Diethyl 2-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate (17b): Yield 75% (after column chromatography with chloroform–methanol mixtures (100:1 and 50:1, v/v)). A yellow oil; IR (film): ν = 3443, 3356, 3233, 3147, 3063, 2986, 1698, 1661, 1626, 1220, 1027, 784, 752 cm−1; 1H-NMR (200 MHz, CDCl3): δ = 8.26 (dd, J = 7.2 Hz, J = 1.1 Hz, 1H, Haromat.), 7.98 (d, J = 2.4 Hz, 1H, Haromat.), 7.86 (dd, J = 8.3 Hz, J = 1.1 Hz, 1H, Haromat.), 7.70 (s, 1H, HC5′), 7.54 (dd, J = 8.3 Hz, J = 7.2 Hz, 1H, Haromat.), 7.22 (d, J = 2.4 Hz, 1H, Haromat.), 5.46 (s, 2H, CH2), 4.62–4.49 (m, 2H, PCH2CH2), 4.30 (s, 2H, NH2), 4.12–3.97 (m, 4H, 2 × POCH2CH3), 2.47–2.30 (m, 2H, PCH2), 1.26 (t, J = 7.2 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 164.13, 163.84, 145.81, 143.92, 133.37, 131.85, 127.25, 126.92, 123.70, 123.02, 122.26, 122.19, 122.02, 113.90, 62.12 (d, J = 6.4 Hz, POC), 44.49 (PCC), 35.15, 27.23 (d, J = 141.4 Hz, PC), 16.32 (d, J = 5.7 Hz, POCC); 31P-NMR (81 MHz, CDCl3): δ = 26.33 ppm. Anal. Calcd. for C21H24N5O5P: C, 55.14; H, 5.29; N, 15.31. Found: C, 55.36; H, 5.06; N, 15.25.
Diethyl 3-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate (17c): Yield 79% (after column chromatography with chloroform–methanol mixtures (100:1 and 50:1, v/v)). A white powder; m.p. 180–182 °C; IR (KBr): ν = 3451, 3343, 3235, 3148, 3067, 2986, 1700, 1652, 1619, 1222, 1029, 794, 759 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.28 (dd, J = 7.3 Hz, J = 0.8 Hz, 1H, Haromat.), 8.00 (d, J = 2.3 Hz, 1H, Haromat.), 7.87 (dd, J = 8.2 Hz, J = 0.8 Hz, 1H, Haromat.), 7.70 (s, 1H, HC5′), 7.56 (dd, J = 8.2 Hz, J = 7.3 Hz, 1H, Haromat.), 7.23 (d, J = 2.3 Hz, 1H, Haromat.), 5.49 (s, 2H, CH2), 4.42 (t, J = 6.9 Hz, 2H, PCH2CH2CH2), 4.33 (s, 2H, NH2), 4.11–4.04 (m, 4H, 2 × POCH2CH3), 2.21 (dqu, J = 18.6 Hz, J = 6.9 Hz, 2H, PCH2CH2), 1.73 (dt, J = 18.6 Hz, J = 7.7 Hz, 2H, PCH2), 1.30 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, CDCl3): δ = 164.01, 163.83, 148.37, 143.72, 134.07, 132.14, 127.43, 126.05, 123.71, 122.96, 122.33, 122.16, 122.15, 112.40, 61.52 (d, J = 6.7 Hz, POC), 49.77 (d, J = 15.2 Hz, PCCC), 35.68, 23.83 (d, J = 3.5 Hz, PCC), 22.32 (d, J = 143.2 Hz, PC), 16.65 (d, J = 5.5 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 30.01 ppm. Anal. Calcd. for C22H26N5O5P: C, 56.05; H, 5.56; N, 14.86. Found: C, 55.80; H, 5.41; N, 14.60.
Diethyl 4-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate (17d): Yield 70% (after column chromatography with chloroform–methanol mixtures (100:1 and 50:1, v/v)). A yellow powder; m.p. 218–220 °C; IR (KBr): ν = 3451, 3343, 3235, 3148, 3067, 2986, 1700, 1652, 1619, 1222, 1029, 794, 759 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.28 (dd, J = 7.3 Hz, J = 0.8 Hz, 1H, Haromat.), 8.00 (d, J = 2.3 Hz, 1H, Haromat.), 7.87 (dd, J = 8.2 Hz, J = 0.8 Hz, 1H, Haromat.), 7.70 (s, 1H, HC5′), 7.56 (dd, J = 8.2 Hz, J = 7.3 Hz, 1H, Haromat.), 7.23 (d, J = 2.3 Hz, 1H, Haromat.), 5.49 (s, 2H, CH2), 4.42 (t, J = 6.9 Hz, 2H, PCCCCH2),4.33 (s, 2H, NH2), 4.20–4.00 (m, 4H, 2 × POCH2CH3), 2.02 (qu, J = 6.9 Hz, 2H, PCCCH2), 1.80–1.60 (m, 4H, PCCH2 and PCH2), 1.30 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 163.99, 163.81, 148.39, 143.61, 134.08, 132.15, 127.42, 126.04, 123.55, 122.97, 122.34, 122.17, 121.14, 112.38, 61.26 (d, J = 6.0 Hz, 2 × POC), 49.16, 35.67, 30.64 (d, J = 16.0 Hz, PCCC), 24.28 (d, J = 138.9 Hz, PC), 19.62 (d, J = 4.7 Hz, PCC), 16.69 (d, J = 5.7 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 30.91 ppm. Anal. Calcd. for C23H28N5O5P: C, 56.90; H, 5.81; N, 14.43. Found: C, 56.95; H, 5.75; N, 14.69.
Diethyl 2-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate (17e): Yield 80% (after crystallization from a methanol–diethyl ether mixture). A white powder; m.p. 170–172 °C; IR (KBr): ν = 3420, 3330, 3233, 3154, 2982, 1700, 1650, 1620, 1228, 1049, 1016, 797, 757 cm−1; 1H-NMR (600 MHz, DMSO-d6): δ = 8.10 (d, J = 7.3 Hz, 1H, Haromat.), 8.05 (d, J = 8.2 Hz, 1H, Haromat.), 8.00 (s, 1H, HC5′), 7.99 (d, J = 2.3 Hz, 1H, Haromat.), 7.63 (dd, J = 8.2 Hz, J = 7.3 Hz, 1H, Haromat.), 7.30 (d, J = 2.3 Hz, 1H, Haromat.), 6.00 (s, 2H, NH2), 5.28 (s, 2H, CH2), 4.52 (dt, J = 14.2 Hz, J = 3.7 Hz, 1H, PCCHaHb), 4.38 (ddd, J = 14.2 Hz, J = 10.1 Hz, J = 7.0 Hz, 1H, PCCHaHb), 4.23–4.18 (m, 1H, PCH), 4.07–4.00 (m, 4H, 2 × POCH2CH3), 1.21 (t, J = 7.0 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 163.35, 163.30, 143.25, 137.05, 134.17, 131.67, 129.82, 127.07, 126.51, 123.20, 122.98, 122.19, 122.04, 121.04, 120.83, 120.51, 67.57 (d, J = 168.4 Hz, PC), 63.36 (d, J = 6.8 Hz, POC), 63.24 (d, J = 6.8 Hz, POC), 52.46 (d, J = 11.7 Hz, PCC), 34.41, 16.60 (d, J = 4.7 Hz, 2 × POCC); 31P-NMR (243 MHz, DMSO-d6): δ = 25.85 ppm. Anal. Calcd. for C21H24N5O6P: C, 53.28; H, 5.11; N, 14.79. Found: C, 53.55; H, 5.35; N, 14.59.
Diethyl 3-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate (17f): Yield 75%. (after crystallization from a methanol–diethyl ether mixture). An orange powder; m.p. 222–224 °C; IR (KBr): ν = 3437, 3333, 3234, 3140, 3068, 3034, 2922, 1653, 1616, 1223, 1049, 778, 744 cm−1; 1H-NMR (600 MHz, DMSO-d6): δ = 8.10 (d, J = 6.9 Hz, 1H, Haromat.), 8.06 (d, J = 8.1 Hz, 1H, Haromat.), 8.00 (d, J = 2.2 Hz, 1H, Haromat.), 7.93 (s, 1H, HC5′), 7.63 (dd, J = 8.1 Hz, J = 6.9 Hz, 1H, Haromat.), 7.31 (t, J = 2.2 Hz, 1H, Haromat.), 6.00 (brs, 2H, NH2), 5.35 (brs, 1H, OH), 5.29 (s, 2H, CH2), 4.45 (dd, J = 13.8 Hz, J = 3.6 Hz, 1H, PCCCHaHb), 4.26 (dd, J = 13.8 Hz, J = 7.7 Hz, 1H, PCCCHaHb), 4.15–4.09 (m, 1H, PCCH), 4.02–3.93 (m, 4H, 2 × POCH2CH3), 1.97 (ddd, J = 18.0 Hz, J = 15.4 Hz, J = 5.3 Hz, 1H, PCHaHb), 1.88 (ddd, J = 18.0 Hz, J = 15.4 Hz, J = 7.1 Hz, 1H, PCHaHb), 1.21 (t, J = 7.0 Hz, 3H, POCH2CH3), 1.20 (t, J = 7.0 Hz, 3H, POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 164.02, 163.84, 148.39, 143.29, 134.09, 132.16, 127.45, 126.05, 124.65, 122.98,122.34, 122.18, 121.14, 112.39, 65.45 (d, J = 1.8 Hz, PCC), 61.62 (d, J = 6.4 Hz, POC), 61.42 (d, J = 6.4 Hz, POC), 55.92 (d, J = 13.0 Hz, PCCC), 35.62, 31.47 (d, J = 137.0 Hz, PC), 16.66 (d, J = 5.9 Hz, 2 × POCC); 31P-NMR (243 MHz, DMSO-d6): δ = 27.89 ppm. Anal. Calcd. for C22H26N5O6P: C, 54.21; H, 5.38; N, 14.37. Found: C, 53.92; H, 5.29; N, 14.15.
Diethyl 2-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy-methylphosphonate (17g): Yield 77% (after column chromatography with chloroform–methanol mixtures (100:1 or 50:1, v/v)). A yellow powder; m.p. 221–223 °C; IR (KBr): ν = 3438, 3339, 3233, 3145, 2980, 2890, 1701, 1651, 1620, 1222, 1048, 1024, 791, 743 cm−1; 1H-NMR (600 MHz, DMSO-d6): δ = 8.10 (d, J = 7.2 Hz, 1H, Haromat.), 8.05 (d, J = 8.2 Hz, 1H, Haromat.), 8.00 (d, J = 2.2 Hz, 1H, Haromat.), 7.96 (s, 1H, HC5′), 7.63 (dd, J = 8.2 Hz, J = 7.2 Hz, 1H, Haromat.), 7.31 (t, J = 2.2 Hz, 1H, Haromat.), 6.00 (brs, 2H, NH2), 5.29 (s, 2H, CH2), 4.50 (t, J = 5.0 Hz, 2H, PCH2OCH2CH2), 3.92–3.88 (m, 6H, 2 × POCH2CH3, PCOCH2CH2), 3.80 (d, J = 8.3 Hz, 2H, PCH2O), 1.12 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 164.04, 163.80, 148.40, 143.56, 134.09, 132.17, 127.44, 126.06, 123.97, 122.97, 122.35, 122.17, 121.15, 112.40, 71.00 (d, J = 11.6 Hz, PCOC), 64.31 (d, J = 163.1 Hz, PC), 62.16 (d, J = 6.3 Hz, POC), 49.47, 35.63, 16.63 (d, J = 5.3 Hz, 2 × POCC); 31P-NMR (243 MHz, DMSO-d6): δ = 20.69 ppm. Anal. Calcd. for C22H26N5O6P: C, 54.21; H, 5.38; N, 14.37. Found: C, 54.38; H, 5.46; N, 14.54.
Diethyl 2-(2-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}ethoxy)ethylphosphonate (17h): Yield 77% (after column chromatography with chloroform–methanol mixtures (100:1 or 50:1, v/v)). An orange powder; m.p. 180–183 °C; IR (KBr): ν = 3434, 3353, 3230, 2981, 2927, 1698, 1659, 1623, 1222, 1025, 787, 748 cm−1; 1H-NMR (600 MHz, CDCl3): δ = 8.33 (d, J = 7.2 Hz, 1H, Haromat.), 8.06 (d, J = 2.2 Hz, 1H, Haromat.), 7.82 (d, J = 8.2 Hz, 1H, Haromat.), 7.78 (s, 1H, HC5′), 7.54 (dd, J = 8.2 Hz, J = 7.2 Hz,1H, Haromat.), 7.18 (d, J = 2.2 Hz, 1H, Haromat.), 6.00 (brs, 2H, NH2), 5.49 (s, 2H, CH2), 4.59 (t, J = 5.1 Hz, 2H, PCH2CH2OCH2CH2), 4.19–4.02 (m, 4H, 2 × POCH2CH3), 3.78 (t, J = 5.1 Hz, 2H, PCCOCH2CH2), 3.65 (dt, J = 15.4 Hz, J = 6.9 Hz, 2H, PCH2CH2O), 2.16 (dt, J = 18.6 Hz, J = 6.9 Hz, 2H, PCH2CH2O), 1.35 (t, J = 7.1 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 164.02, 163.84, 148.38, 143.56, 134.08, 132.13, 127.41, 126.03, 124.00, 123.00, 122.33, 122.20, 121.16, 112.37, 68.69, 64.73(PCCO), 61.36 (d, J = 6.4 Hz, POC), 49.71, 35.68, 26.28 (d, J = 137.2 Hz, PC), 16.64 (d, J = 5.6 Hz, POCC); 31P-NMR (243 MHz, CDCl3): δ = 27.84 ppm. Anal. Calcd. for C23H28N5O6P: C, 55.09; H, 5.63; N, 13.97. Found: C, 55.12; H, 5.36; N, 13.70.
Diethyl 2-{4-[(5-Amino-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)methyl]-1H-1,2,3-triazol-1-yl}acetamido-methylphosphonate (17i): Yield 96%. A yellow powder; m.p. 222–224 °C; IR (KBr): ν = 3447, 3374, 3225, 3146, 2991, 2927, 1695, 1649, 1619, 1223, 1021, 779, 744 cm−1; 1H-NMR (600 MHz, DMSO-d6): δ = 8.73 (t, J = 5.5 Hz, 1H, NHCO), 8.10 (d, J = 7.2 Hz, 1H, Haromat.), 8.05 (d, J = 8.1 Hz, 1H, Haromat.), 8.00 (d, J = 2.2 Hz, 1H, Haromat.), 7.95 (s, 1H, HC5′), 7.63 (dd, J = 8.1 Hz, J = 7.2 Hz, 1H, Haromat.), 7.31 (t, J = 2.2 Hz, 1H, Haromat.), 6.00 (brs, 2H, NH2), 5.30 (s, 2H, CH2), 5.09 (s, 2H), 4.02–3.97 (m, 4H, 2 × POCH2CH3), 3.60 (dd, J = 11.8 Hz, J = 5.9 Hz, 2H, PCH2NH), 1.19 (t, J = 6.9 Hz, 6H, 2 × POCH2CH3); 13C-NMR (151 MHz, DMSO-d6): δ = 165.97 (d, J = 4.5 Hz, C=O), 164.00, 163.82, 148.39, 143.40, 134.08, 132.19, 127.45, 126.08, 125.18, 122.93, 122.35, 122.13, 121.12, 112.43, 62.32 (d, J = 6.2 Hz, POC), 51.83, 35.58, 34.62 (d, J = 155.2 Hz, PC), 16.64 (d, J = 5.7 Hz, POCC); 31P-NMR (121.5 MHz, DMSO-d6): δ = 22.29 ppm. Anal. Calcd. for C22H25N6O6P: C, 52.80; H, 5.04; N, 16.79. Found: C, 52.71; H, 4.86; N, 16.53.