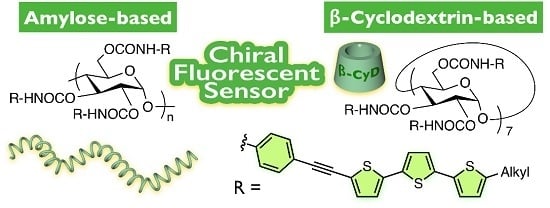
Development of Amylose- and β-Cyclodextrin-Based Chiral Fluorescent Sensors Bearing Terthienyl Pendants
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.3. Sample Preparation for Fluorescence Measurements
3.4. Preparation of HPLC Columns
3.5. Instruments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Ariëns, E.J. Stereochemistry: A source of problems in medicinal chemistry. Med. Res. Rev. 1986, 6, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B. Pharmacologically active compounds in the environment and their chirality. Chem. Soc. Rev. 2010, 39, 4466–4503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, L.-S.; Zeng, S. Stereoselectivity of chiral drug transport: A focus on enantiomer–transporter interaction. Drug Metab. Rev. 2014, 46, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kotake, M.; Sakan, T.; Nakamura, N.; Senoh, S. Resolution into optical isomers of some amino acids by paper chromatography. J. Am. Chem. Soc. 1951, 73, 2973–2974. [Google Scholar] [CrossRef]
- Hess, H.; Burger, G.; Musso, H. Complete enantiomer separation by chromatography on potato starch. Angew. Chem. Int. Ed. Engl. 1978, 17, 612–614. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yashima, E. Polysaccharide derivatives for chromatographic separation of enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1021–1043. [Google Scholar] [CrossRef]
- Yashima, E. Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation. J. Chromatogr. A 2001, 906, 105–125. [Google Scholar] [CrossRef]
- Ikai, T.; Okamoto, Y. Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chem. Rev. 2009, 109, 6077–6101. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Ikai, T.; Moro, M.; Maeda, K.; Kanoh, S. Synthesis of polysaccharide derivatives bearing pyridine N-oxide groups and their use as asymmetric organocatalysts. React. Funct. Polym. 2011, 71, 1055–1058. [Google Scholar] [CrossRef]
- Ikai, T.; Kimura, K.; Maeda, K.; Kanoh, S. Synthesis of polysaccharide serivatives bearing bromobenzoate pendants for use as chiral auxiliaries. React. Funct. Polym. 2014, 82, 52–57. [Google Scholar] [CrossRef]
- Ikai, T.; Suzuki, D.; Kojima, Y.; Yun, C.; Maeda, K.; Kanoh, S. Chiral fluorescent sensors based on cellulose derivatives bearing terthienyl pendants. Polym. Chem. 2016, 7, 4793–4801. [Google Scholar] [CrossRef]
- Pu, L. Fluorescence of organic molecules in chiral recognition. Chem. Rev. 2004, 104, 1687–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, L.; Liang, W.; Gui, J.; Xu, D.; Wu, W.; Nakai, Y.; Nishijima, M.; Fukuhara, G.; Mori, T.; et al. Inherently chiral azonia[6]helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in water. J. Org. Chem. 2016, 81, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Qu, K.; Ren, J.; Qu, X. Chiral detection using reusable fluorescent amylose-functionalized graphene. Chem. Sci. 2011, 2, 2050–2056. [Google Scholar] [CrossRef]
- Vogt, U.; Zugenmaier, P. Presented at the European Science Foundation Workshop on Specific Interaction in Polysaccharide Systems; European Science Foundation: Uppsala, Sweden, 1983. [Google Scholar]
- Yamamoto, C.; Yashima, E.; Okamoto, Y. Structural analysis of amylose tris(3,5-dimethylphenylcarbamate) by NMR relevant to its chiral recognition mechanism in HPLC. J. Am. Chem. Soc. 2002, 124, 12583–12589. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; Chapter 8. [Google Scholar]
- Aburatani, R.; Okamoto, Y.; Hatada, K. Optical resolving ability of 3,5-dimethylphenylcarbamates of oligosaccharides and cyclodextrins. Bull. Chem. Soc. Jpn. 1990, 63, 3606–3610. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Useful chiral packing materials for high-performance liquid chromatographic resolution of enantiomers: Phenylcarbamates of polysaccharides coated on silica gel. J. Am. Chem. Soc. 1984, 106, 5357–5359. [Google Scholar] [CrossRef]
- Under the chromatographic conditions used, the interactions between Am-1b and the amino acid derivatives (6–10) were very weak and their k1 values were estimated to be nearly zero. Therefore, we could not discuss the resolution ability of Am-1b for 6–10.
- Okamoto, Y.; Kawashima, M.; Yamamoto, K.; Hatada, K. Useful chiral packing materials for high-performance liquid chromatographic resolution. Cellulose triacetate and tribenzoate coated on macroporous silica gel. Chem. Lett. 1984, 13, 739–742. [Google Scholar] [CrossRef]
- Okamoto, Y.; Aburatani, R.; Hatada, K. Chromatographic chiral resolution. XIV. Cellulose tribenzoate derivatives as chiral stationary phases for high-performance liquid chromatography. J. Chromatogr. A 1987, 389, 95–102. [Google Scholar] [CrossRef]
- Koller, H.; Rimböck, K.-H.; Mannschreck, A. High-pressure liquid chromatography on triacetylcellulose: Characterization of a sorbent for the separation of enantiomers. J. Chromatogr. A 1983, 282, 89–94. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.








| Racemates | Am-1 | ATPC | ||
|---|---|---|---|---|
| k1 | α | k1 | α | |
| 11a | 1.15 (R) | 1.17 | 1.40 (R) | ca. 1 |
| 11b | 0.29 | 1.0 | 0.95 (R) | 1.22 |
| 11c | 0.14 | 1.0 | 0.39 | 1.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikai, T.; Yun, C.; Kojima, Y.; Suzuki, D.; Maeda, K.; Kanoh, S. Development of Amylose- and β-Cyclodextrin-Based Chiral Fluorescent Sensors Bearing Terthienyl Pendants. Molecules 2016, 21, 1518. https://doi.org/10.3390/molecules21111518
Ikai T, Yun C, Kojima Y, Suzuki D, Maeda K, Kanoh S. Development of Amylose- and β-Cyclodextrin-Based Chiral Fluorescent Sensors Bearing Terthienyl Pendants. Molecules. 2016; 21(11):1518. https://doi.org/10.3390/molecules21111518
Chicago/Turabian StyleIkai, Tomoyuki, Changsik Yun, Yutaka Kojima, Daisuke Suzuki, Katsuhiro Maeda, and Shigeyoshi Kanoh. 2016. "Development of Amylose- and β-Cyclodextrin-Based Chiral Fluorescent Sensors Bearing Terthienyl Pendants" Molecules 21, no. 11: 1518. https://doi.org/10.3390/molecules21111518