Characteristics of the Protoporphyrin IX Binding Sites on Human Serum Albumin Using Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of the PpIX-HSA Complex
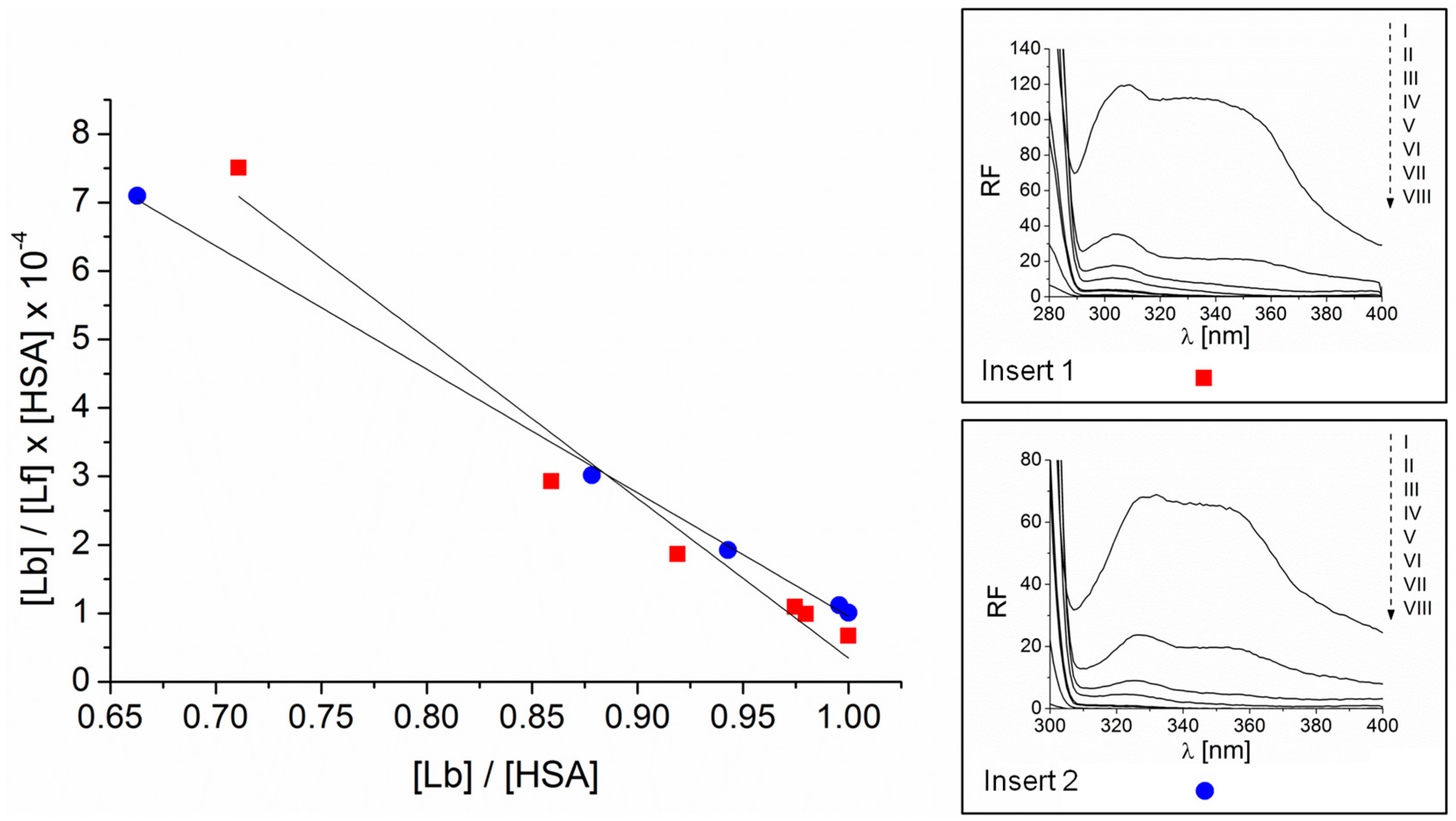
2.2. Association and Stern–Volmer Constants
2.3. Molecular Properties of PpIX
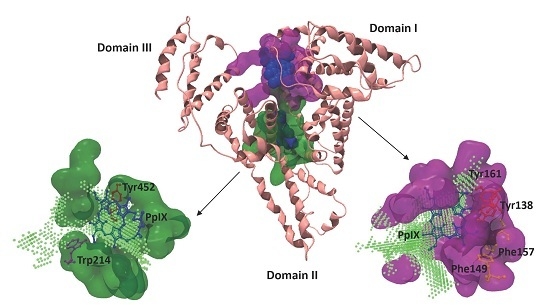
2.4. Visualization of the PpIX Binding Sites in the HSA Molecule
2.5. Binding Site Interactions between PpIX and HSA Amino Acids
3. Materials and Methods
3.1. Chemicals
3.2. Absorption UV-VIS Analysis
3.3. Fluorescence Analysis
3.4. Association Constants and Quenching Constants
3.5. Computational Simulation
3.6. Molecular Docking Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brancaleon, L.; Moseley, H. Effects of photoproducts on the binding properties of protoporphyrin IX to proteins. Biophys. Chem. 2002, 96, 77–87. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The inhibition of ferrochelatase enhances 5-aminolevulinic acid-based photodynamic action for prostate cancer. Photodiagnosis Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Huang, J. Determination of cytochrome c and other heme proteins using the reduction wave of mercury protoporphyrin IX groups generated by a hydroxylamine induced replacement reaction. Anal. Chem. 2009, 81, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- IdrishMiah, M. Correlation of protoporphyrin metabolism in cell proliferation with embryonic development time: A spectroscopic investigation. Optik 2009, 120, 980–983. [Google Scholar] [CrossRef]
- Rinco, O.; Brenton, J.; Douglas, A.; Maxwell, A.; Henderson, M.; Indrelie, K.; Wessels, J.; Widin, J. The effect of porphyrin structure on binding to human serum albumin by fluorescence spectroscopy. J. Photochem. Photobiol. A Chem. 2009, 208, 91–96. [Google Scholar] [CrossRef]
- Chow, J.M.; Huang, G.C.; Lin, H.Y.; Shen, S.C.; Yang, L.Y.; Chen, Y.C. Cytotoxic effects of metal protoporphyrins in glioblastoma cells: Roles of albumin, reactive oxygen species, and heme oxygenase-1. Toxicol. Lett. 2008, 177, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Kobuchi, H.; Munetomo, K.; Fujita, H.; Yamamoto, M.; Utsumi, T.; Inoue, K.; Shuin, T.; Sasaki, J.; Inoue, M.; et al. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Moll. Cell. Biochem. 2011, 358, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, E.; Sou, K.; Nakagawa, A.; Sakai, H.; Komatsu, T.; Kobayashi, K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugatechemistry. Bioconjug. Chem. 2009, 20, 1419–1440. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Z.; Saberi, M.R.; Chamani, J. Determination of LMF binding site on a HSA-PPIX complex in the presence of human holo transferrin from the viewpoint of drug loading on proteins. PLoS ONE 2014, 9, e84045. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ishikawa, N.; Komatsu, T. Human serum albumin-based peroxidase having an iron protoporphyrin IX in artificial heme pocket. Chem.-Asian J. 2012, 7, 2534–2537. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Lee, S.S.; Kiserow, D.J.; McGown, L.B. Enzyme solubilization in a reversed micellar microreactor with a bile salt cosurfactant. J. Colloid Interface Sci. 1997, 193, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Maciążek-Jurczyk, M.; Sułkowska, A.; Bojko, B.; Równicka-Zubik, J.; Sułkowski, W.W. A spectroscopic study of phenylbutazone and aspirin bound to serum albumin in rheumatoid diseases. Spectrochim. Acta A 2011, 82, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M. Protoporphyrin IX: Some recent research. Acc. Chem. Res. 1979, 12, 374–381. [Google Scholar] [CrossRef]
- Crossley, M.J.; Harding, M.M.; Sternhell, S. Tautomerism in 2-substituted 5,10,15,20-tetraphenylporphyrins. J. Am. Chem. Soc. 1986, 108, 3608–3613. [Google Scholar] [CrossRef]
- Caughey, W.S.; Ibers, J.A. Crystal and molecular structure of the free base porphyrin, protoporphyrin IX dimethyl ester. J. Am. Chem. Soc. 1977, 99, 6639–6645. [Google Scholar] [CrossRef] [PubMed]
- Storm, C.B.; Teklu, Y.; Sokoloski, E.A. N-H tautomerism in porphyrins and chlorins. Ann. N. Y. Acad. Sci. 1973, 206, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Otagiri, M. A molecular functional study on the interactions of drugs with plasma proteins. Drug. Metab. Pharmacokinet. 2005, 20, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Wardell, M.; Wang, Z.; Ho, J.X.; Robert, J.; Ruker, F.; Ruble, J.; Carter, D.C. The atomic structure of human methemalbumin at 1.9 Å. Biochem. Biophys. Res. Commun. 2002, 291, 813–819. [Google Scholar] [CrossRef] [PubMed]
- CLC Drug Discovery Workbench version 1.5; CLC Bio, a QIAGEN Company: Aarhus, Denmark, 2014.
- Dessault Systemes BIOVIA. Discovery Studio Modeling Environment; Release 2017; Dessault Systemes: San Diego, CA, USA, 2016. [Google Scholar]
- Hiratsuka, T. Conformational changes in the 23-kilodalton NH2-terminal peptide segment of myosin ATPase associated with ATP hydrolysis. J. Biol. Chem. 1990, 265, 18786–18790. [Google Scholar] [PubMed]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org (accessed on 7 October 2014).
- ChemBioOffice Ultra version 13.02.3021, 2012; CambridgeSoft: Cambridge, MA, USA, 1986–2012.
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.P. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Marvin 14.9.29.0, ChemAxon. 2014. Available online: http://www.chemaxon.com (accessed on 2 February 2014).
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced Protein-Ligand docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound PpIX and PpIX-HSA complexes are available (in Mol Files) from the authors.

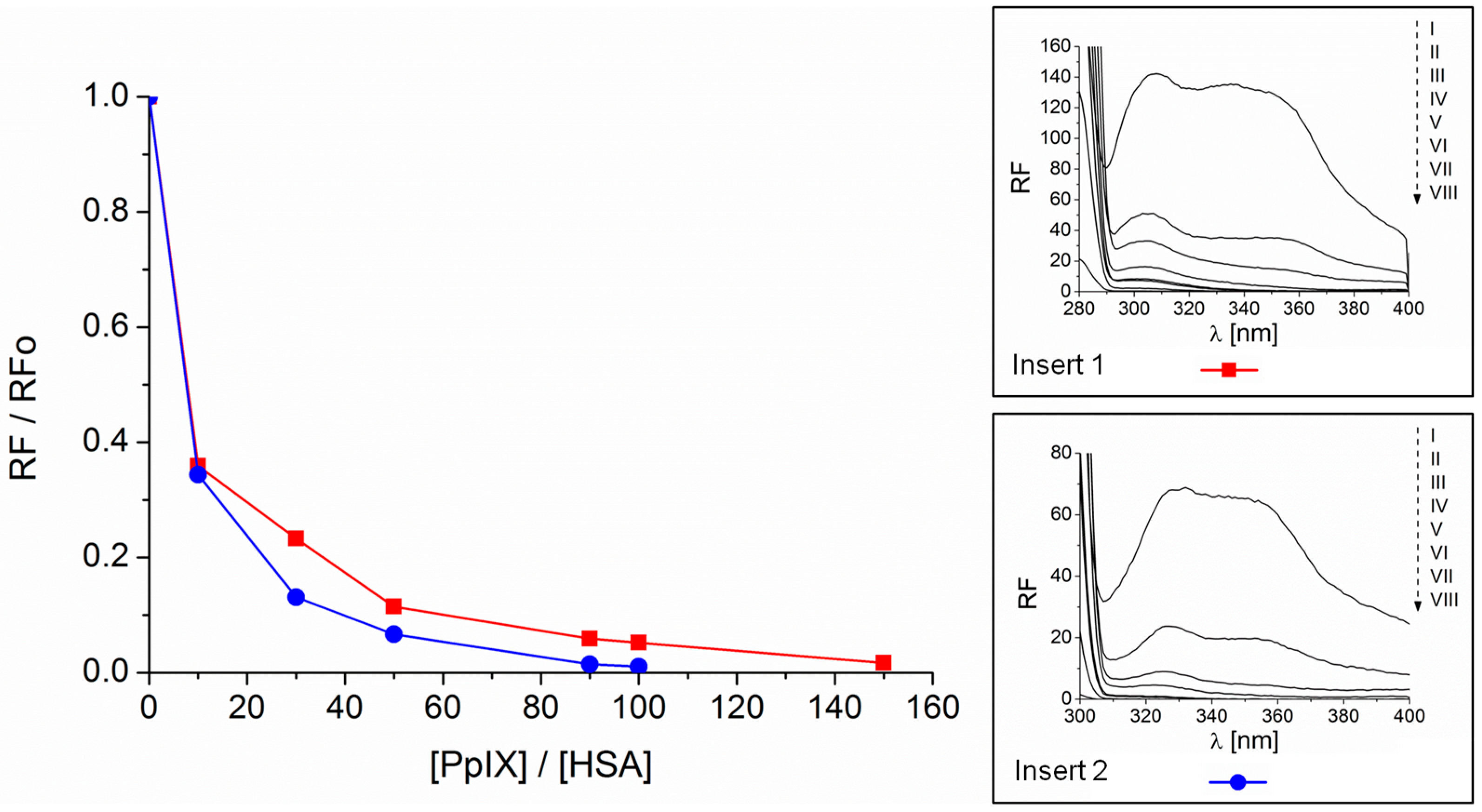












| λex = 280 nm | λex = 295 nm | |
|---|---|---|
| PpIX-HSA | Ka = 1.68 × 105 M−1 n = 1 | Ka = 2.30 × 105 M−1 n = 1 |
| KS-V = 1.78 × 105 M−1 | KS-V = 1.93 × 105 M−1 |
| Binding Sites in HSA | PpIX-HSA Complexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Volume (Å3) | Radius (Å) | Location | Domain | Subdomain | α-Helix | Energy (a.u.) a | No. of H-bonds | Common Interacting Residues with PpIX |
| 1 | 787.46 | 15 | Core | I | B | h1, h2, h3, h4 | −73.55 | 4 (6) b | Arg 117, Tyr 138, Ile 142, His 146, Phe 149, Phe 157, Tyr 161, Arg 186 |
| 2 | 420.35 | 13 | Core | I II III | B A B A | h4 h1, h2 h3 h3, h4 | −70.34 | 3 (5) b | Ala 191, Ala 194, Lys 195, Leu 198, Trp 214, Arg 218, Val 343, Lys 436, Lys 444, Pro 447, Cys 448, Asp 451, Tyr 452, Val 455 |
| 3 | 165.38 | 11 | Surface | II III | A B A | h1, h2 h2, h3 h5, h6 | −11.42 | 0 (2) b | Phe 206, Arg 209, Ala 210, Lys 351 |
| 4 | 146.94 | 10 | Surface | I | A B | h2, h3 h2, h3 | −48.25 | 2 (4) b | Glu 17, Ala 21, Ala 158, Lys 159, Lys 162 |
| 5 | 65.02 | 10 | Surface | III | A B | h1, h2 h2, h3 | −44.90 | 2 (4) b | Leu 394, Leu 398, Ala 406, Arg 410, Lys 413, Thr 540, Lys 541 |
| 6 | 52.22 | 10 | Surface | III | B | h1, h2 | −33.56 | 1 (3) b | Pro 421, His 510, Thr 506, Phe 509, His 510, Lys 524, Ala 528 |
| 7 | 52.22 | 10 | Surface | III | A | h2, h5, h6 | −49.05 | 2 (4) b | Met 87, Gln 32, Gln 33, Lys 466 |
| Hydrogen Bonding Interactions | |||||||
| Site 1 | Site 2 | ||||||
| PDB atom name | Ligand atom | Type of contact | Distance (Å) | PDB atom name | Ligand atom | Type of contact | Distance (Å) |
| (D) Arg117:HN (bb) | (A) PpIX:O(pp) | Conventional | 2.02 * | (D) Lys444:HZ1 (sc) | (A) PpIX:O (pp) | Conventional | 2.01 * |
| (D) Arg117:HE (sc) | (A) PpIX:O (pp) | Conventional | 2.77 * | (D) Lys444:HZ1 (sc) | (A) PpIX:O (pp) | Conventional | 2.25 * |
| (D) Tyr161:HH (sc) | (A) PpIX:O (pp) | Conventional | 2.00 * | (D) Lys444:HZ2 (sc) | (A) PpIX:O (pp) | Conventional | 2.58 * |
| (D) Arg186:HE (sc) | (A) PpIX: (r) | π-Donor Non classical | 3.01 * | (D) PpIX:NH (r) (A) PpIX:N (r) | Conventional Intramolecular | 2.21 * | |
| (D) PpIX:NH (r) (A) PpIX:N (r) | Conventional Intramolecular | 2.21 * | (D) PpIX:NH (r) (A) PpIX:N (r) | Conventional Intramolecular | 2.24 * | ||
| (D) PpIX:NH (r) (A) PpIX:N (r) | Conventional Intramolecular | 2.24 * | |||||
| Hydrophobic Interactions | |||||||
| Site 1 | Site 2 | ||||||
| PDB atom name | Ligand atom | Type of contact | Distance (Å) | PDB atom name | Ligand atom | Type of contact | Distance (Å) |
| Tyr138 (sc) | PpIX:C(v) | π-Alkyl | 5.05 | Ala191 (sc) | PpIX:C (v) | Alkyl | 4.09 |
| Ile142 (sc) | PpIX:C (m) | Alkyl | 4.52 | Ala191 (sc) | PpIX:C (m) | Alkyl | 4.10 |
| Ile142 (sc) | PpIX:C (v) | Alkyl | 4.57 | Ala194 (sc) | PpIX:C (v) | Alkyl | 3.90 |
| Ile142:CD1 (sc) | PpIX (pr) | π-σ | 3.15 | Lys195 (sc) | PpIX:C (v) | Alkyl | 4.00 |
| His146 (sc) | PpIX:C( m) | π-σ | 3.62 | Lys195 (sc) | PpIX (pr) | π-Alkyl | 4.88 |
| His146 (sc) | PpIX:C (v) | π-Alkyl | 5.10 | Leu198 (sc) | PpIX:C (m) | Alkyl | 5.14 |
| Phe149 (sc) | PpIX:C (m) | π-Alkyl | 4.83 | Trp214 (sc) | PpIX:C (v) | π-Alkyl | 4.49 |
| Phe157 (sc) | PpIX:C (v) | π-Alkyl | 4.50 | Trp214 (sc) | PpIX:C (v) | π-Alkyl | 4.61 |
| Arg186 (sc) | PpIX (chms) | π-Alkyl | 3.11 | Arg218 (sc) | PpIX:C (v) | Alkyl | 4.55 |
| Arg186 (sc) | PpIX (pr) | π-Alkyl | 3.46 | Val343 (sc) | PpIX:C (v) | Alkyl | 3.49 |
| Arg186 (sc) | PpIX (pr) | π-Alkyl | 4.35 | Lys436 (sc) | PpIX:C (m) | Alkyl | 5.31 |
| Arg186 (sc) | PpIX (pr) | π-Alkyl | 4.72 | Lys436 (sc) | PpIX:C (m) | Alkyl | 3.79 |
| Arg186 (sc) | PpIX (pr) | π-Alkyl | 5.42 | Lys436 (sc) | PpIX (pr) | π-Alkyl | 5.27 |
| Pro447 (sc) | PpIX (pr) | π-Alkyl | 5.21 | ||||
| Lys190 (sc) | PpIX:C (v) | Alkyl | 4.43 | Pro447:C,O (bb) Cys448:N (bb) | PpIX (pr) | Amide π-Stacked | 3.94 |
| Lys190 (sc) | PpIX:C (m) | Alkyl | 4.72 | Cys448 (sc) | PpIX (pr) | π-Alkyl | 5.32 |
| Lys190:CD1 (sc) | PpIX (pr) | π-σ | 3.94 | Cys448 (sc) | PpIX (chms) | π-Alkyl | 5.33 |
| Lys190 (sc) | PpIX (pr) | π-Alkyl | 5.27 | Asp451:C,O (bb) Tyr452:N (bb) | PpIX (pr) | Amide π-Stacked | 4.47 |
| Asp451:C,O (bb) Tyr452:N (bb) | PpIX (chms) | Amide π-Stacked | 5.66 | ||||
| Tyr452 (sc) | PpIX (pr) | π-π T-shaped | 4.96 | ||||
| Tyr452 (sc) | PpIX (m) | π-Alkyl | 4.59 | ||||
| Tyr452 (sc) | PpIX (v) | π-Alkyl | 5.48 | ||||
| Val455 (sc) | PpIX:C (m) | Alkyl | 4.56 | ||||
| Val455 (sc) | PpIX:C (v) | Alkyl | 3.97 | ||||
| Val455 (sc) | PpIX (pr) | π-Alkyl | 5.43 | ||||
| Miscellaneous Interactions | Unfavorable Interactions | ||||||
| Site 2 | Site 2 | ||||||
| PDB atom name | Ligand atom | Type of contact | Distance (Å) | PDB atom name | Ligand atom | Type of contact | Distance (Å) |
| Cys448:SG (sc) | PpIX (pr) | π-Sulfur | 3.76 | Lys436:HZ2 (sc) | PpIX:C (m) | Steric Bumps | 1.04 |
| Lys436:HZ3 (sc) | PpIX:H (m) | Steric Bumps | 1.34 | ||||
| Lys436:NZ (sc) | PpIX:H (m) | Steric Bumps | 1.37 | ||||
| Lys436:HZ2 (sc) | PpIX:C (m) | Steric Bumps | 1.64 | ||||
| Lys436:NZ (sc) | PpIX:C (m) | Steric Bumps | 2.20 | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułkowski, L.; Pawełczak, B.; Chudzik, M.; Maciążek-Jurczyk, M. Characteristics of the Protoporphyrin IX Binding Sites on Human Serum Albumin Using Molecular Docking. Molecules 2016, 21, 1519. https://doi.org/10.3390/molecules21111519
Sułkowski L, Pawełczak B, Chudzik M, Maciążek-Jurczyk M. Characteristics of the Protoporphyrin IX Binding Sites on Human Serum Albumin Using Molecular Docking. Molecules. 2016; 21(11):1519. https://doi.org/10.3390/molecules21111519
Chicago/Turabian StyleSułkowski, Leszek, Bartosz Pawełczak, Mariola Chudzik, and Małgorzata Maciążek-Jurczyk. 2016. "Characteristics of the Protoporphyrin IX Binding Sites on Human Serum Albumin Using Molecular Docking" Molecules 21, no. 11: 1519. https://doi.org/10.3390/molecules21111519