Hydrodechlorination of Tetrachloromethane over Palladium Catalysts Supported on Mixed MgF2-MgO Carriers
Abstract
:1. Introduction
2. Results and Discussion
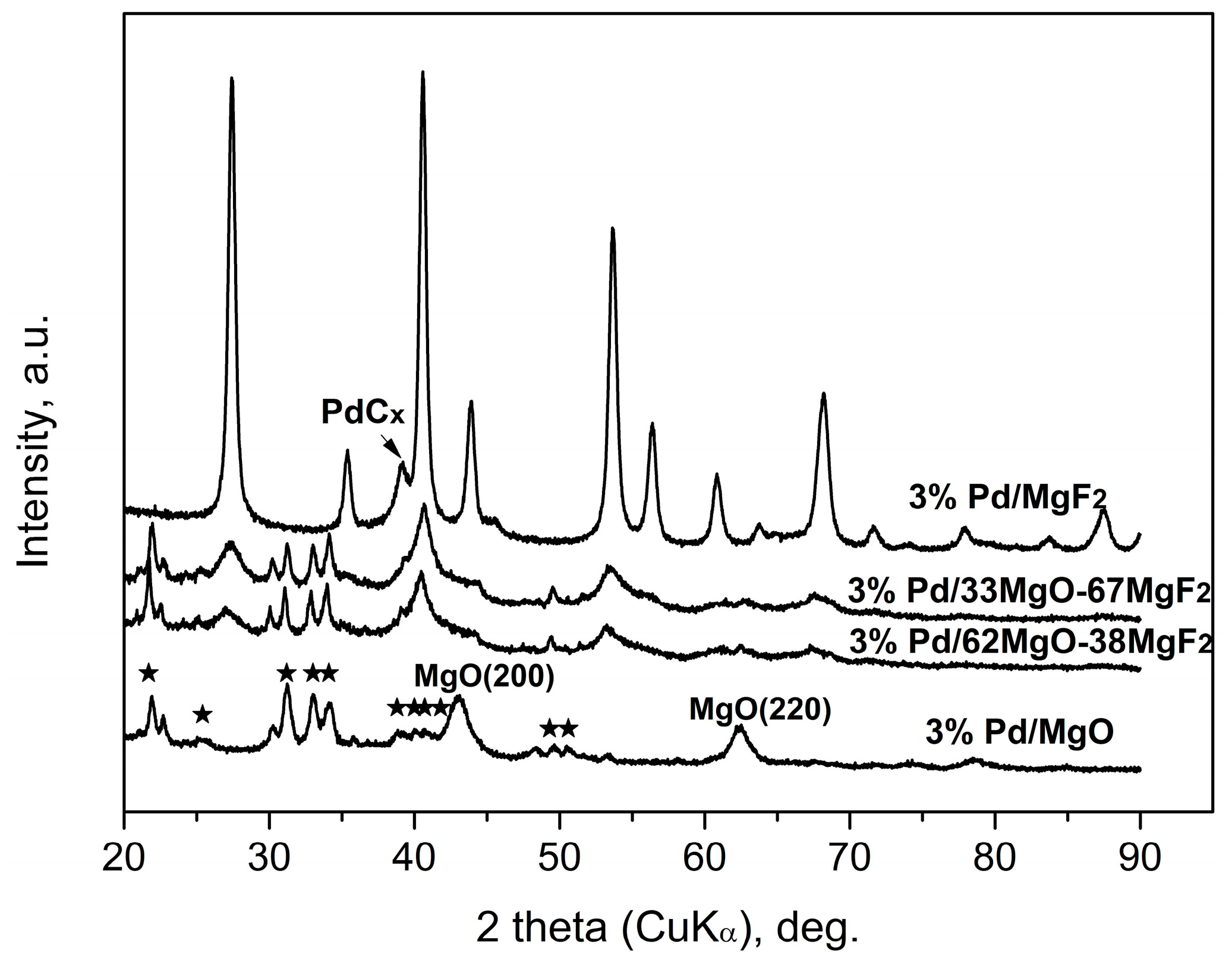
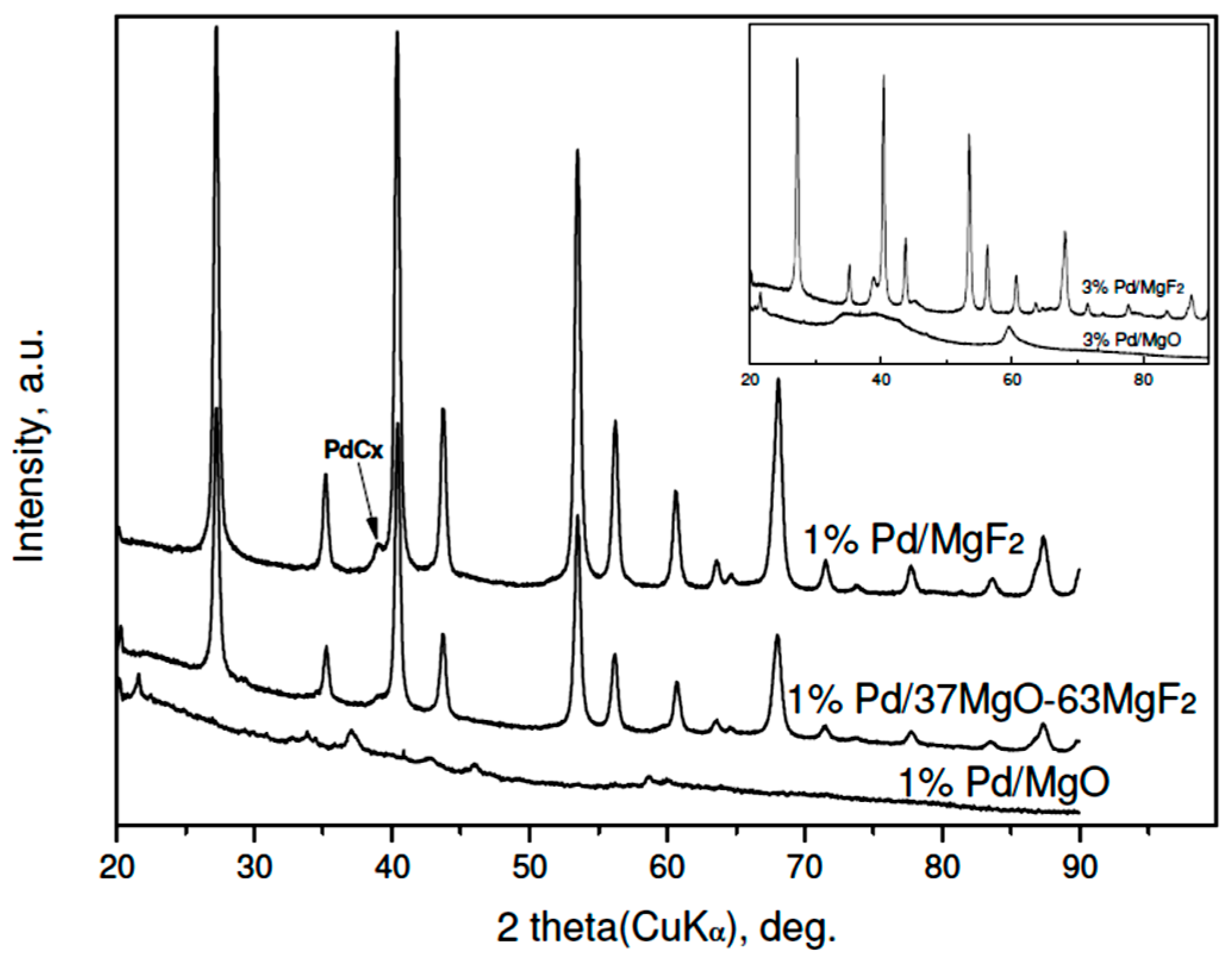
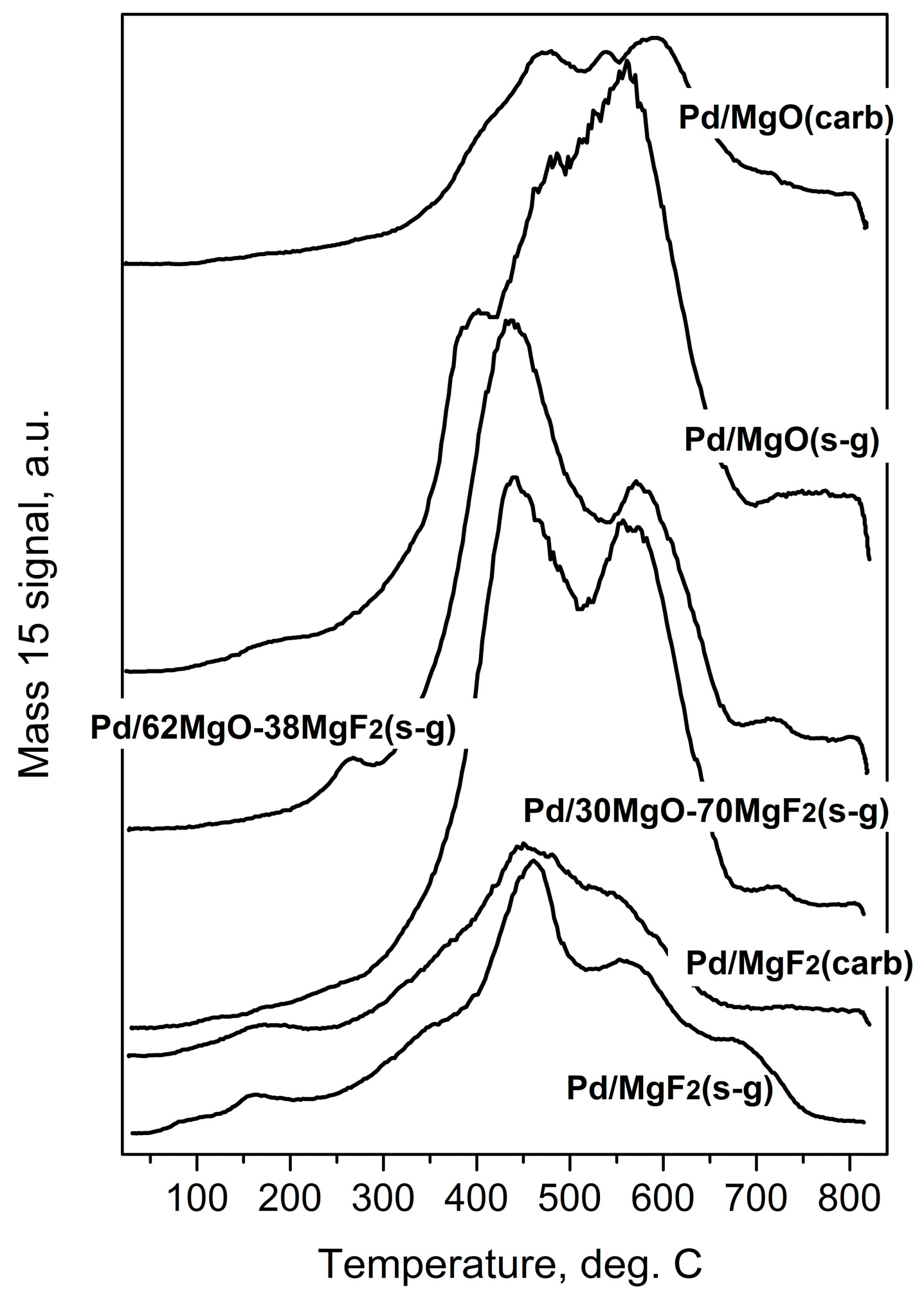
2.1. Catalyst Characterization
2.2. Catalytic Performance of Pd/MgO-MgF2 in CCl4 Hydrodechlorination
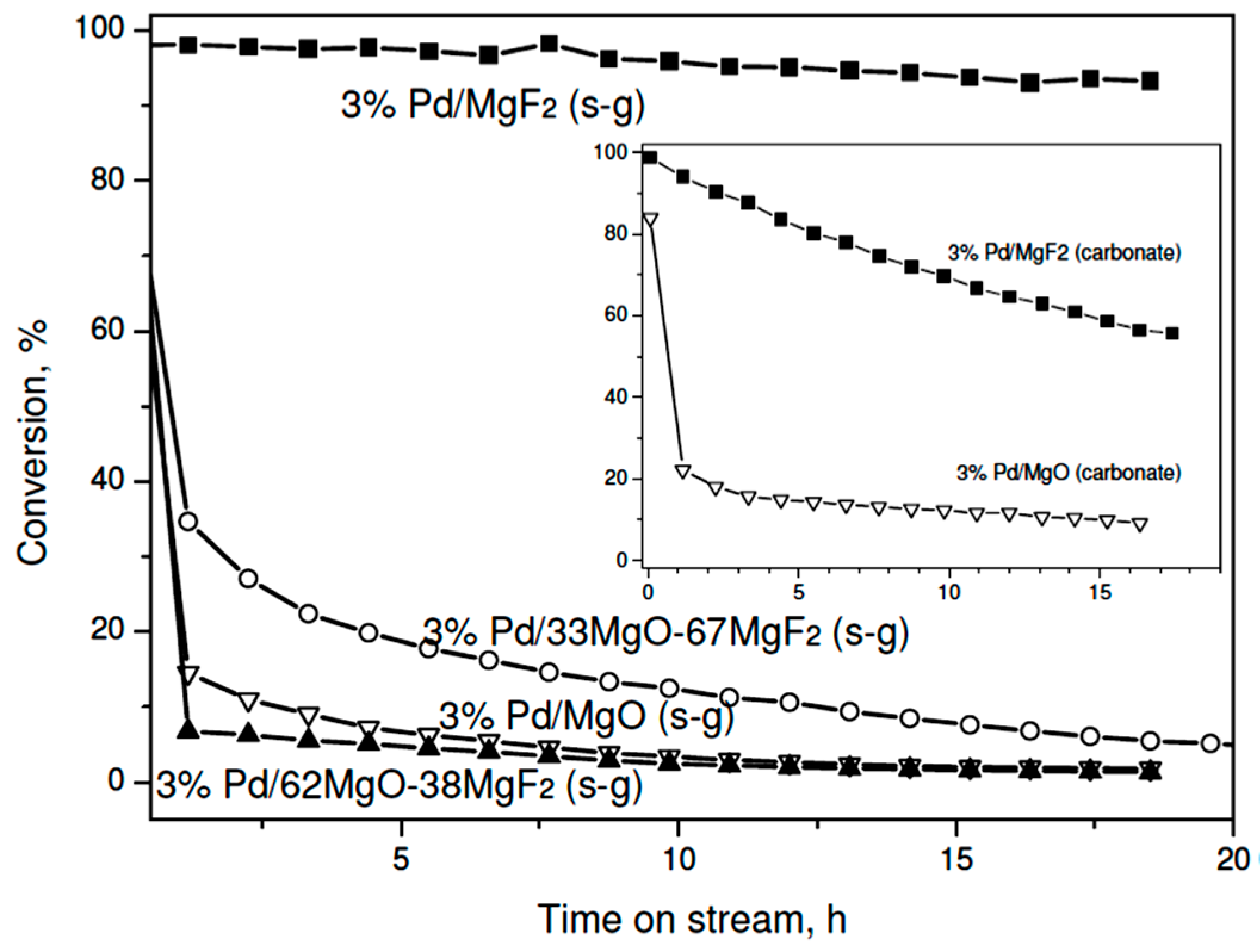
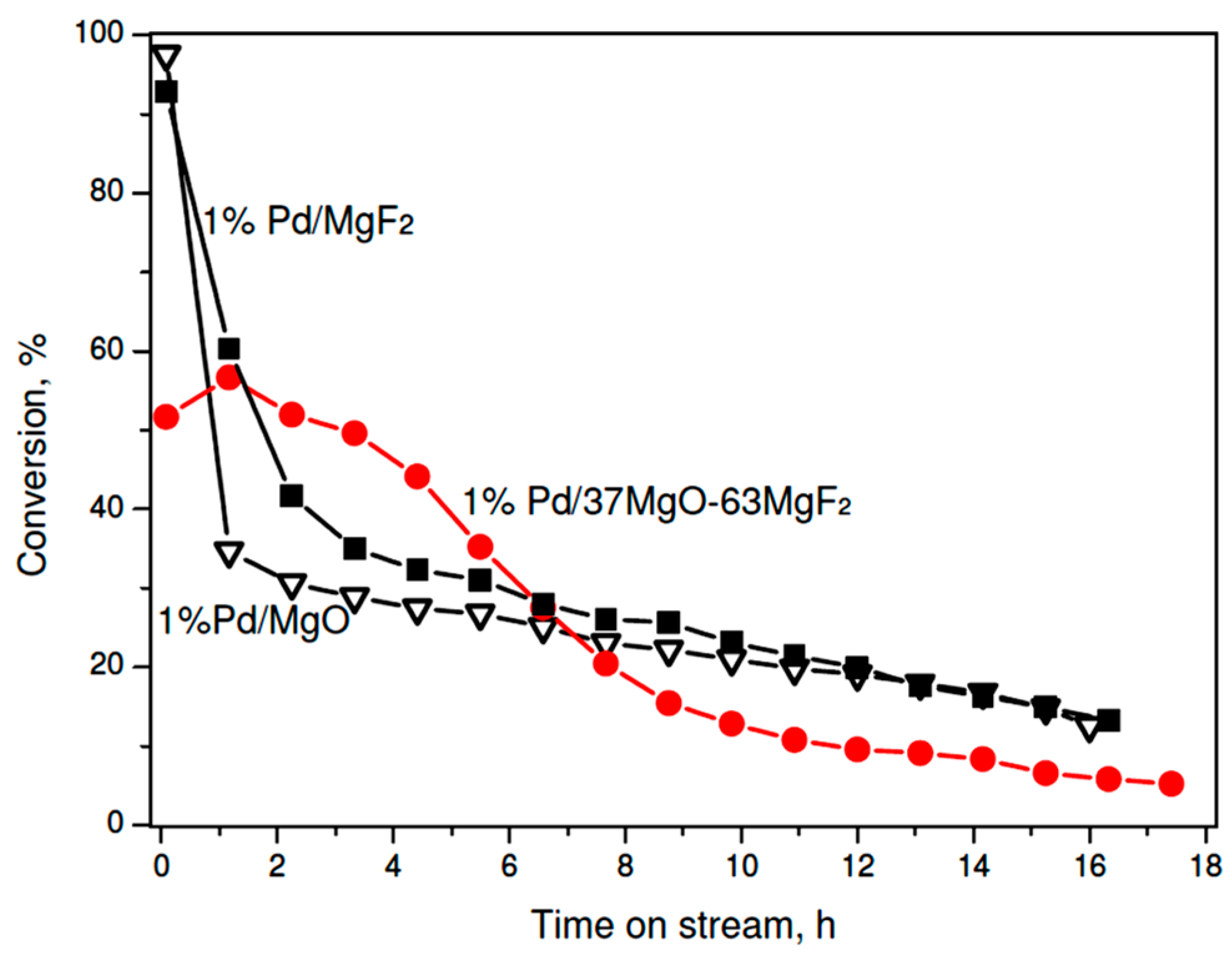
2.2.1. Catalytic Activity
2.2.2. Product Selectivities and Activation Energies
2.2.3. Working State of MgO-MgF2 Supported Palladium Catalyst
3. Experimental Section
3.1. Preparation of MgO-MgF2 Supports
3.2. Preparation of MgO-MgF2 Supported Palladium Catalysts
3.3. Characterization of Catalysts
3.4. Hydrodechlorination of Tetrachloromethane
Author Contributions
Conflicts of Interest
References
- Kovalchuk, V.I.; d’Itri, J.L. Catalytic chemistry of chloro- and chlorofluorocarbon dehalogenation: From macroscopic observations to molecular level understanding. Appl. Catal. A Gen. 2004, 271, 13–25. [Google Scholar] [CrossRef]
- Keane, M.A. Supported Transition Metal Catalysts for Hydrodechlorination Reactions. ChemCatChem 2011, 3, 800–821. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Beard, B.C. Genesis of durable catalyst for selective hydrodechlorination of CCl4 to CHCl3. Appl. Catal. A 1998, 174, 33–39. [Google Scholar] [CrossRef]
- Coq, B.; Cognion, J.-M.; Figuéras, F.; Tournigant, D. Conversion Under Hydrogen of Dichlorodifluoromethane over Supported Palladium Catalysts. J. Catal. 1993, 141, 21–33. [Google Scholar] [CrossRef]
- Coq, B.; Figuéras, F.; Hub, S.; Tournigant, D. Effect of the Metal-Support Interaction on the Catalytic Properties of Palladium for the Conversion of Difluorodichloromethane with Hydrogen: Comparison of Oxides and Fluorides as Supports. J. Phys. Chem. 1995, 99, 11159–11166. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Berndt, H.; Bozorg Zadeh, H.; Kemnitz, E.; Nickkho-Amiry, M.; Pohl, M.; Skapin, T.; Winfield, J.M. The properties of platinum or palladium supported on β-aluminium trifluoride or magnesium difluoride: Catalysts for the hydrodechlorination of 1,1-dichlorotetrafluoroethane. J. Mater. Chem. 2002, 12, 3499–3507. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Sachtler, W.M.H. Determination by X-ray photoelectron spectroscopy of the electronic state of Pd clusters in Y zeolite. J. Chem. Soc. Faraday Trans. 1991, 87, 3703–3708. [Google Scholar] [CrossRef]
- Coq, B.; Medina, F.; Tichit, D.; Morato, A. The catalytic transformation of chlorofluorocarbons in hydrogen on metal-based catalysts supported on inorganic fluorides. Catal. Today 2004, 88, 127–137. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M.; Bedia, J.; Arevalo-Bastante, A.; Rodriguez, J.J. Enhanced activity of carbon-supported Pd–Pt catalysts in the hydrodechlorination of dichloromethane. Appl. Catal. B 2016, 184, 55–63. [Google Scholar] [CrossRef]
- Diaz, E.; Mohedano, A.F.; Casas, J.A.; Shalaby, C.; Eser, S.; Rodriguez, J.J. On the performance of Pd and Rh catalysts over different supports in the hydrodechlorination of the MCPA herbicide. Appl. Catal. B 2016, 186, 151–156. [Google Scholar] [CrossRef]
- Alvarez-Montero, M.A.; Gomez-Sainero, L.M.; Martin-Martinez, M.; Heras, F.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with Pd on activated carbon catalysts for the treatment of residual gas streams. Appl. Catal. B 2010, 96, 148–156. [Google Scholar] [CrossRef]
- Gomez-Sainero, L.M.; Seoane, X.L.; Fierro, J.L.G.; Arcoya, A. Liquid-Phase Hydrodechlorination of CCl4 to CHCl3 on Pd/Carbon Catalysts: Nature and Role of Pd Active Species. J. Catal. 2002, 209, 279–288. [Google Scholar] [CrossRef]
- Babu, N.S.; Lingaiah, N.; Pasha, N.; Kumar, J.V.; Prasad, P.S.S. Influence of particle size and nature of Pd species on the hydrodechlorination of chloroaromatics: Studies on Pd/TiO2 catalysts in chlorobenzene conversion. Catal. Today 2009, 141, 120–124. [Google Scholar] [CrossRef]
- Ding, E.; Jujjuri, S.; Sturgeon, M.; Shore, S.G.; Keane, M.A. Novel one step preparation of silica supported Pd/Sr and Pd/Ba catalysts via an organometallic precursor: Application in hydrodechlorination and hydrogenation. J. Mol. Catal. A 2008, 294, 51–60. [Google Scholar] [CrossRef]
- Cobo, M.I.; Conesa, J.A.; de Correa, C.M. The Effect of NaOH on the Liquid-Phase Hydrodechlorination of Dioxins over Pd/γ-Al2O3. J. Phys. Chem. A 2008, 112, 8715–8722. [Google Scholar] [CrossRef] [PubMed]
- Jujjuri, S.; Ding, E.; Hommel, E.L.; Shore, S.G.; Keane, M.A. Synthesis and characterization of novel silica-supported Pd/Yb bimetallic catalysts: Application in gas-phase hydrodechlorination and hydrogenation. J. Catal. 2006, 239, 486–500. [Google Scholar] [CrossRef]
- Early, K.; Kovalchuk, V.I.; Lonyi, F.; Deshmukh, S.; d’Itri, J.L. Hydrodechlorination of 1,1-Dichlorotetrafluoroethane and Dichlorodifluoromethane Catalyzed by Pd on Fluorinated Aluminas: The Role of Support Material. J. Catal. 1999, 182, 219–227. [Google Scholar] [CrossRef]
- Álvarez-Montero, M.A.; Rodriguez, J.J.; Gómez-Sainero, L.M. Platinum Nanoparticles Supported on Activated Carbon Catalysts for the Gas-phase Hydrodechlorination of Dichloromethane: Influence of Catalyst Composition and Operating Conditions. Nanomater. Nanotechnol. 2016, 6, 18. [Google Scholar] [CrossRef]
- Choi, H.C.; Choi, S.H.; Yang, O.B.; Lee, J.S.; Lee, K.H.; Kim, Y.G. Hydrodechlorination of Carbon Tetrachloride over Pt/MgO. J. Catal. 1996, 161, 790–797. [Google Scholar] [CrossRef]
- Dal Santo, V.; Dossi, C.; Recchia, S.; Colavita, P.E.; Vlaic, G.; Psaro, R. Carbon tetrachloride hydrodechlorination with organometallics-based platinum and palladium catalysts on MgO. J. Mol. Catal. A 2002, 182–183, 157–166. [Google Scholar] [CrossRef]
- Aytam, H.P.; Akula, V.; Janmanchi, K.; Kamaraju, S.R.R.; Panja, K.R.; Gurram, K.; Niemantsverdriet, J.W. Characterization and Reactivity of Pd/MgO and Pd/γ-Al2O3 Catalysts in the Selective Hydrogenolysis of CCl2F2. J. Phys. Chem. B 2002, 106, 1024–1031. [Google Scholar] [CrossRef]
- Clarke, J.K.A.; Bradley, M.I.; Garvie, L.A.J.; Craven, A.J.; Baird, T. Pt/MgO as Catalyst for Hydrogenolysis Reactions of C5 and C6 Hydrocarbons: Evidence for Metal-Support Interactions. J. Catal. 1993, 143, 122–137. [Google Scholar] [CrossRef]
- Padmasri, A.H.; Venugopal, A.; Siva Kumar, V.; Shashikala, V.; Nagaraja, B.M.; Seetharamulu, P.; Sreedhar, B.; David Raju, B.; Kanta Rao, P.; Rama Rao, K.S. Role of hydrotalcite precursors as supports for Pd catalysts in hydrodechlorination of CCl2F2. J. Mol. Catal. A 2004, 223, 329–337. [Google Scholar] [CrossRef]
- Malinowski, A.; Juszczyk, W.; Pielaszek, J.; Bonarowska, M.; Wojciechowska, M.; Karpiński, Z. Magnesium fluoride as a catalytic support in hydrodechlorination of CCl2F2 (CFC-12). Chem. Commun. 1999, 685–686. [Google Scholar] [CrossRef]
- Patil, P.T.; Dimitrov, A.; Kirmse, H.; Neumann, W.; Kemnitz, E. Non-aqueous sol–gel synthesis, characterization and catalytic properties of metal fluoride supported palladium nanoparticles. Appl. Catal. B 2008, 78, 80–91. [Google Scholar] [CrossRef]
- Cao, Y.C.; Jiang, X.Z. Supported platinum–gallium catalysts for selective hydrodechlorination of CCl4. J. Mol. Catal. A 2005, 242, 119–128. [Google Scholar] [CrossRef]
- Cao, Y.C.; Li, Y. In situ synthesis of supported palladium complexes: Highly stable and selective supported palladium catalysts for hydrodechlorination of CCl2F2. Appl. Catal. A 2005, 294, 298–305. [Google Scholar] [CrossRef]
- Murthy, J.K.; Chandra Shekar, S.; Padmasri, A.H.; Venugopal, A.; Siva Kumar, V.; Nagaraja, B.M.; Shashikala, V.; David Raju, B.; Kanta Rao, P.; Rama Rao, K.S. Promotional effect of magnesia addition to active carbon supported Pd catalyst on the characteristics and hydrodechlorination activity of CCl2F2. Catal. Commun. 2004, 5, 161–167. [Google Scholar] [CrossRef]
- Wuttke, S.; Coman, S.M.; Kröhnert, J.; Jentoft, F.C.; Kemnitz, E. Sol–gel prepared nanoscopic metal fluorides—A new class of tunable acid–base catalysts. Catal. Today 2010, 152, 2–10. [Google Scholar] [CrossRef]
- Prescott, H.A.; Li, Z.-J.; Kemnitz, E.; Deutsch, J.; Lieske, H. New magnesium oxide fluorides with hydroxy groups as catalysts for Michael additions. J. Mater. Chem. 2005, 15, 4616–4628. [Google Scholar] [CrossRef]
- Zieliński, M.; Wojciechowska, M. Preparation of MgF2-MgO supports with specified acid-base properties, and their influence on nickel catalyst activity in toluene hydrogenation. Stud. Surf. Sci. Catal. 2010, 175, 429–432. [Google Scholar]
- Zieliński, M. The catalytic and physico-chemical properties of Ni/MgF2–MgO catalysts. Appl. Catal. A 2012, 449, 15–22. [Google Scholar] [CrossRef]
- Bonarowska, M.; Pielaszek, J.; Juszczyk, W.; Karpiński, Z. Characterization of Pd–Au/SiO2 Catalysts by X-ray Diffraction, Temperature-Programmed Hydride Decomposition, and Catalytic Probes. J. Catal. 2000, 195, 304–315. [Google Scholar] [CrossRef]
- Pinna, F.; Signoretto, M.; Strukul, I.G.; Polizzi, S.; Pernicone, N. Pd-SiO2 catalysts. stability of β-PdHx as a function of Pd dispersion. React. Kinet. CataI. Lett. 1997, 60, 9–13. [Google Scholar] [CrossRef]
- Nag, N.K. A Study on the Formation of Palladium Hydride in a Carbon-Supported Palladium Catalyst. J. Phys. Chem. B 2001, 105, 5945–5949. [Google Scholar] [CrossRef]
- Ichikawa, S.; Poppa, H.; Boudart, M. Disproportionation of CO on small particles of silica-supported palladium. J. Catal. 1985, 91, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Beard, B.C. Agglomeration of Pt particles in the presence of chlorides. Appl. Catal. A 1998, 188, 229–240. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, I.G.; Lee, J.S.; Lee, K.H.; Jang, E.J. Hydrodechlorination of CCl4 over Pt/Al2O3: Effects of platinum particle size on product distribution. Appl. Catal. A 2003, 240, 129–142. [Google Scholar] [CrossRef]
- Bae, J.W.; Lee, J.S.; Lee, K.H. Hydrodechlorination of CCl4 over Pt/γ-Al2O3 prepared from different Pt precursors. Appl. Catal. A 2008, 334, 156–167. [Google Scholar] [CrossRef]
- Shin, E.-J.; Spiller, A.; Tavoularis, G.; Keane, M.A. Chlorine–nickel interactions in gas phase catalytic hydrodechlorination: Catalyst deactivation and the nature of reactive hydrogen. Phys. Chem. Chem. Phys. 1999, 1, 3173–3181. [Google Scholar] [CrossRef]
- Ordonez, S.; Díez, F.V.; Sastre, H. Characterisation of the deactivation of platinum and palladium supported on activated carbon used as hydrodechlorination catalysts. Appl. Catal. B 2001, 31, 113–122. [Google Scholar] [CrossRef]
- Frankel, K.A.; Jang, B.W.-L.; Spivey, J.J.; Roberts, G.W. Deactivation of hydrodechlorination catalysts: I. Experiments with 1,1,1-trichloroethane. Appl. Catal. A 2001, 205, 263–278. [Google Scholar] [CrossRef]
- Frankel, K.A.; Jang, B.W.-L.; Roberts, G.W.; Spivey, J.J. Deactivation of hydrodechlorination catalysts: II—Experiments with 1,1-dichloroethylene and 1,1-dichloroethane. Appl. Catal. A 2001, 209, 401–413. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, H.C.; Yang, O.B.; Lee, K.H.; Lee, J.C.; Kim, J.G. Hydrodechlorination of tetrachloromethane over supported Pt catalysts. J. Chem. Soc. Chem. Commun. 1995, 2169–2170. [Google Scholar] [CrossRef]
- Legawiec-Jarzyna, M.; Śrębowata, A.; Juszczyk, W.; Karpiński, Z. Hydrodechlorination over Pd–Pt/Al2O3 catalysts: A comparative study of chlorine removal from dichlorodifluoromethane, carbon tetrachloride and 1,2-dichloroethane. Appl. Catal. A 2004, 271, 61–68. [Google Scholar] [CrossRef]
- Bonarowska, M.; Kaszkur, Z.; Łomot, D.; Rawski, M.; Karpiński, Z. Effect of gold on catalytic behavior of palladium catalysts in hydrodechlorination of tetrachloromethane. Appl. Catal. B 2015, 162, 45–56. [Google Scholar] [CrossRef]
- Moon, D.J.; Chung, M.J.; Park, K.Y.; Hong, S.I. Deactivation of Pd catalysts in the hydrodechlorination of chloropentafluoroethane. Appl. Catal. A 1998, 168, 159–170. [Google Scholar] [CrossRef]
- Juszczyk, W.; Malinowski, A.; Karpiński, Z. Hydrodechlorination of CCl2F2 (CFC-12) over γ-alumina supported palladium catalysts. Appl. Catal. A 1998, 166, 311–319. [Google Scholar] [CrossRef]
- Bonarowska, M.; Karpiński, Z.; Kosydar, R.; Szumełda, T.; Drelinkiewicz, A. Hydrodechlorination of CCl4 over carbon-supported palladium–gold catalysts prepared by the reverse “water-in-oil” microemulsion method. C. R. Chim. 2015, 18, 1143–1151. [Google Scholar] [CrossRef]
- Bonarowska, M.; Kaszkur, Z.; Słowik, G.; Ryczkowski, J.; Karpiński, Z. Tetrachloromethane as an Effective Agent to Transform Nanoparticles of Palladium and Gold in Supported Catalysts. ChemCatChem 2016, 8, 2625–2629. [Google Scholar] [CrossRef]
- Fenelonov, V.B.; Mel’gunov, M.S.; Mishakov, I.V.; Richards, R.M.; Chesnokov, V.V.; Volodin, A.M.; Klabunde, K.J. Changes in Texture and Catalytic Activity of Nanocrystalline MgO during Its Transformation to MgCl2 in the Reaction with 1-Chlorobutane. J. Phys. Chem. B 2001, 105, 3937–3941. [Google Scholar] [CrossRef]
- Zieliński, M.; Kiderys, A.; Pietrowski, M.; Tomska-Foralewska, I.; Wojciechowska, M. Synthesis and characterization of new Mg–O–F system and its application as catalytic support. Catal. Commun. 2016, 76, 54–57. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the catalysts and supports are not available from the authors.



| Support | BET Surface Area (m2·g−1) | Average Pore Diameter (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| Sol-Gel Series | |||
| MgO | 152 | 10.5 | 0.39 |
| 62% MgO-38% MgF2 | 208 | 8.2 | 0.43 |
| 33% MgO-67% MgF2 | 144 | 17.8 | 0.64 |
| MgF2 | 32 | 15.5 | 0.12 |
| Carbonate Series | |||
| MgO | 142.7 | 8.19 | 0.29 |
| 37% MgO-63% MgF2 | 51.0 | 23.79 | 0.30 |
| MgF2 | 32.0 | 21.26 | 0.17 |
| Support Series a | Catalyst | Had/Pd From Pulse Chemisorption and Metal Particle Size (d, nm) b | COad/Pd From Static Chemisorption and Metal Particle Size (d, nm) c | H/Pd From TPHD |
|---|---|---|---|---|
| sol-gel | 3% Pd/MgO | 0.509 (2.2) | 0.661 (1.7) | 0.12 |
| 3% Pd/62% MgO-38% MgF2 | 0.495 (2.3) | 0.645 (1.7) | 0.13 | |
| 3% Pd/33% MgO-67% MgF2 | 0.554 (2.0) | 0.672 (1.7) | 0.11 | |
| 3% Pd/MgF2 | 0.175 (6.4) | 0.141 (7.9) | 0.30 | |
| carbonate | 3% Pd/MgO | 0.647 (1.7) | 0.633 (1.8) | 0.11 |
| 3% Pd/MgF2 | 0.098 (11.4) | 0.072 (15.6) | 0.35 | |
| carbonate | 1% Pd/MgO | n.m. d | 0.323 (3.5) | n.m. d |
| 1% Pd/37% MgO-63% MgF2 | n.m. d | 0.317 (3.5) | n.m. d | |
| 1% Pd/MgF2 | n.m. d | 0.259 (4.3) | n.m. d |
| Catalyst | Final Conversion a | Product Distribution a, % | TOF b, s−1 | Activation Energy c, kJ/mol | |||||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2-C5 | CHxCl4x | C2HxCly | C3HxCly | ΣC1-C5 | ||||
| Sol-Gel Series | |||||||||
| 3% Pd/MgO | 1.4 | 20.9 | 15.5 | 3.7 | 52.9 | 7.0 | 36.4 | 1.95 × 10−4 | 56.7 ± 1.4 |
| 3% Pd/62% MgO-38% MgF2 | 1.8 | 17.4 | 19.9 | 11.4 | 43.8 | 7.5 | 37.3 | 2.38 × 10−4 | 50.0 ± 2.1 |
| 3% Pd/33% MgO-67% MgF2 | 4.9 | 16.1 | 23.3 | 8.9 | 47.8 | 3.9 | 39.4 | 6.10 × 10−4 | 51.3 ± 5.2 |
| 3% Pd/MgF2 | 93.4 | 20.5 | 42.2 | 8.8 | 24.5 | 4.0 | 62.7 | 5.55 × 10−2 | 38.3 ± 1.8 |
| Carbonate Series | |||||||||
| 3% Pd/MgO | 9.5 | 16.9 | 25.9 | 11.8 | 38.8 | 6.6 | 42.8 | 1.57 × 10−3 | 48.5 ± 4.6 |
| 3% Pd/MgF2 | 56.1 | 23.9 | 35.1 | 15.9 | 20.5 | 4.6 | 59.0 | 5.36 × 10−2 | 42.1 ± 1.3 |
| 1% Pd/MgO | 13.7 | 17.0 | 10.6 | 7.8 | 62.9 | 1.6 | 27.7 | 2.15 × 10−2 | 65.0 ± 6.2 |
| 1% Pd/37% MgO-63% MgF2 | 5.5 | 26.6 | 22.6 | 3.5 | 40.8 | 6.5 | 49.2 | 5.08 × 10−3 | 50.7 ± 5.6 |
| 1% Pd/MgF2 | 14.1 | 20.6 | 32.9 | 15.9 | 26.3 | 4.3 | 53.5 | 1.51 × 10−2 | 46.3 ± 5.7 |
| Catalyst Sample | Tdes, °C | A | %A | A300/A200 |
|---|---|---|---|---|
| 1 % Pd/37MgO-63MgF2 reduced | 150 | 0.124 | 100.00 | |
| 200 | 0.082 | 66.13 | 0.644 | |
| 250 | 0.059 | 47.58 | ||
| 300 | 0.0528 | 42.58 | ||
| 1 % Pd/37MgO-63MgF2 after HdCl | 150 | 0.337 | 100.00 | |
| 200 | 0.096 | 28.49 | 0.339 | |
| 250 | 0.0325 | 9.64 | ||
| 300 | 0.0254 | 7.54 | ||
| 1 % Pd/MgF2 after HdCl | 150 | 0.0874 | 100.00 | |
| 200 | 0.0414 | 47.37 | 0.360 | |
| 250 | 0.0237 | 27.12 | ||
| 300 | 0.0149 | 17.05 | ||
| 3% Pd/MgO after HdCl | 150 | 0.0049 | 100.00 | |
| 200 | 0.0045 | 91.84 | 0.251 | |
| 250 | 0.00127 | 25.92 | ||
| 300 | 0.00113 | 23.06 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonarowska, M.; Wojciechowska, M.; Zieliński, M.; Kiderys, A.; Zieliński, M.; Winiarek, P.; Karpiński, Z. Hydrodechlorination of Tetrachloromethane over Palladium Catalysts Supported on Mixed MgF2-MgO Carriers. Molecules 2016, 21, 1620. https://doi.org/10.3390/molecules21121620
Bonarowska M, Wojciechowska M, Zieliński M, Kiderys A, Zieliński M, Winiarek P, Karpiński Z. Hydrodechlorination of Tetrachloromethane over Palladium Catalysts Supported on Mixed MgF2-MgO Carriers. Molecules. 2016; 21(12):1620. https://doi.org/10.3390/molecules21121620
Chicago/Turabian StyleBonarowska, Magdalena, Maria Wojciechowska, Maciej Zieliński, Angelika Kiderys, Michał Zieliński, Piotr Winiarek, and Zbigniew Karpiński. 2016. "Hydrodechlorination of Tetrachloromethane over Palladium Catalysts Supported on Mixed MgF2-MgO Carriers" Molecules 21, no. 12: 1620. https://doi.org/10.3390/molecules21121620







