In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of Green Tea and Herbal Tea Extracts on Cell Proliferation
2.2. Induction of Pro-Apoptotic Caspase-3 Activity
2.2.1. Relationship between Pro-Apoptotic Effect and Cell Viability
2.2.2. Morphological Alteration in Normal Cells Associated with Induction of Apoptosis
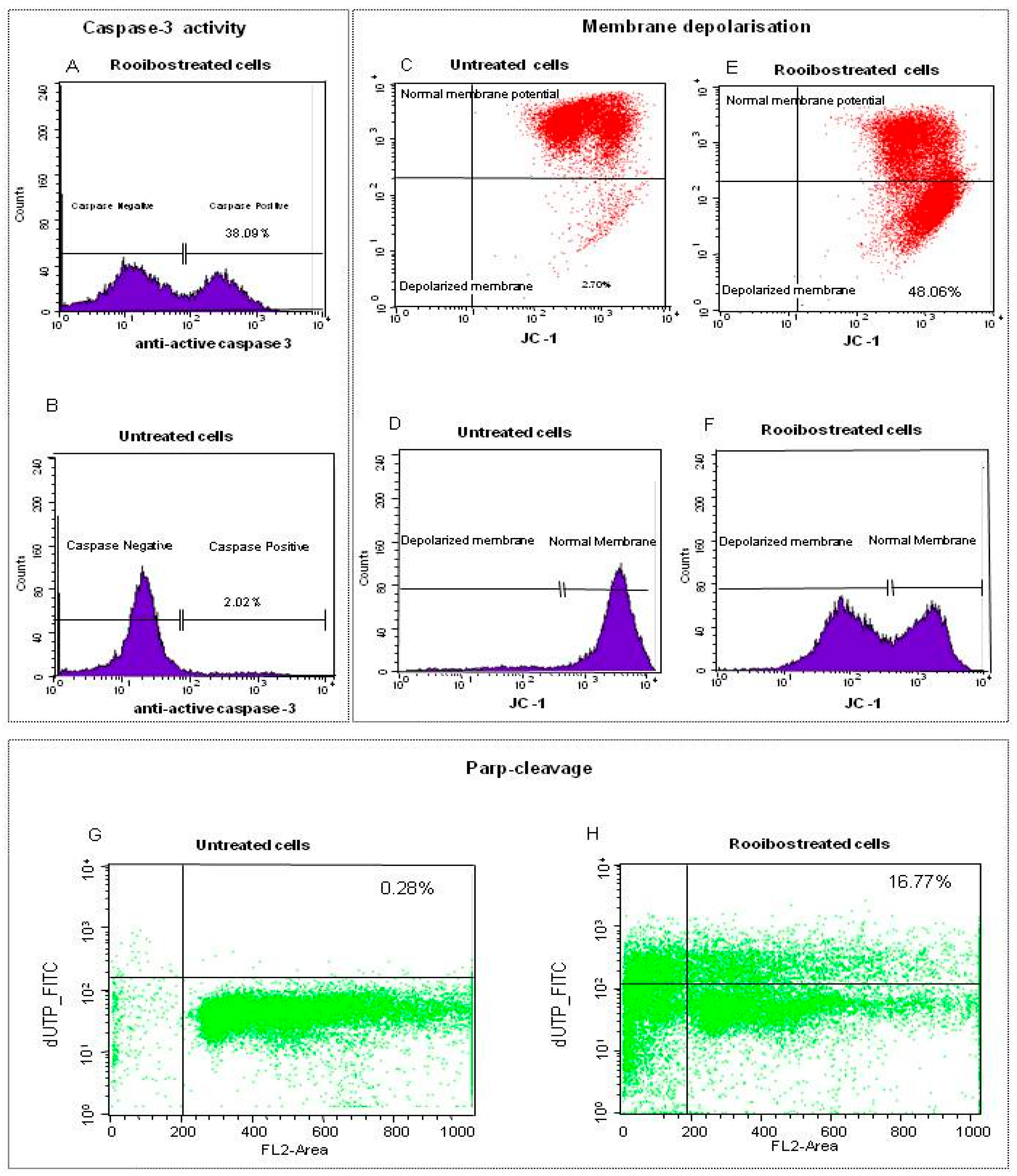
2.2.3. Characterisation of Rooibos-Induced Apoptosis in Normal Cells by Flow Cytometry
2.3. Differences in Polyphenol Content and Specific Ratios between Methanol and Aqueous Extracts
3. Discussion
4. Materials and Methods
4.1. Reagents and Assay Kits
4.2. Preparation of Extracts and Polyphenols Analyses
4.3. Cell Culture
4.4. Modulation of Cell Proliferation and Apoptosis
4.4.1. Cell Proliferation Assay
4.4.2. Cell Viability and Apoptosis Assays
4.4.3. Hoechst Stain
4.5. Characterization of Pro-Apoptotic Activity of Rooibos by Flow Cytometry
4.5.1. Caspase-3 Activity
4.5.2. Membrane Depolarisation
4.5.3. DNA Fragmentation
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, R.H.; Armstrong, A.W. Nonmelanoma skin cancer. Dermatol. Clin. 2012, 30, 125–139. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Skin Cancers. Ultraviolet Radiation and the INTERSUN Programme. Available online: http:/www.who.int/uv/faq/skin cancer/en/index1.html (accessed on 16 November 2016).
- Digiovanni, J. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 1992, 54, 63–128. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Marks, F.; Fürstenberger, G. Proliferative responses of the skin to external stimuli. Environ. Health Perspect. 1993, 101, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E.; Fischer, S.M. Molecular mechanisms of mouse skin tumor promotion. Cancers 2010, 2, 436–482. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Evasion of Apoptosis as a Cellular Stress Response in Cancer. Int. J. Cell Biol. 2010, 2010, 370835. [Google Scholar] [CrossRef] [PubMed]
- Erb, P.; Ji, J.; Kump, E.; Mielgo, A.; Wernli, M. Apoptosis and pathogenesis of melanoma and nonmelanoma skin cancer. Adv. Exp. Med. Biol. 2008, 624, 283–295. [Google Scholar] [PubMed]
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010, 76, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Marques, M.P.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch. Biochem. Biophys. 2011, 508, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Pastore, S.; Dellambra, E.; de Luca, C. New molecular and cellular targets for chemoprevention and treatment of skin tumors by plant polyphenols: A critical review. Curr. Med. Chem. 2013, 20, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.; Traidl-Hoffmann, C. Green tea in dermatology—Myths and facts. J. Dtsch. Dermatol. Ges. 2015, 13, 768–775. [Google Scholar] [CrossRef] [PubMed]
- OyetakinWhite, P.; Tribout, H.; Baron, E. Protective mechanisms of green tea polyphenols in skin. Oxid. Med. Cell Longev. 2012, 2012, 560682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Agarwal, R.; Bickers, D.R.; Mukhtar, H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis 1991, 12, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Huang, M.T.; Ho, C.T.; Chang, R.; Ma, W.; Ferraro, T.; Reuhl, K.R.; Yang, C.S.; Conney, A.H. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992, 52, 6657–6665. [Google Scholar] [PubMed]
- Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Protection against malignant conversion of chemically induced benign skin papillomas to squamous cell carcinomas in SENCAR mice by a polyphenolic fraction isolated from green tea. Cancer Res. 1993, 53, 5409–5412. [Google Scholar] [PubMed]
- Katiyar, S.K.; Mohan, R.R.; Agarwal, R.; Mukhtar, H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis 1997, 18, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Meeran, S.M.; Elmets, C.A.; Katiyar, S.K. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005, 135, 2871–2877. [Google Scholar] [PubMed]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Elmets, C.A.; Agarwal, R.; Mukhtar, H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. J. Photochem. Photobiol. B 1995, 62, 855–861. [Google Scholar] [CrossRef]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.-L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G.S. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. Int. J. Cancer 2005, 114, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Nigam, N.; George, J.; Srivastava, S.; Shukla, Y. Induction of apoptosis by tea polyphenols mediated through mitochondrial cell death pathway in mouse skin tumors. Cancer Biol. Ther. 2009, 13, 1281–1287. [Google Scholar] [CrossRef]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderblom, W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; van der Westhuizen, F.H.; Joubert, E.; Swanevelder, S.; Swart, P.; Gelderblom, W.C. Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem. Toxicol. 2009, 47, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.; Davids, L.M.; Rautenbach, F.; Marnewick, J.L. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J. Photochem. Photobiol. B 2011, 103, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Sissing, L.; Marnewick, J.; de Kock, M.; Swanevelder, S.; Joubert, E.; Gelderblom, W. Effects of South African herbal teas, rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia) on the growth and development of oesophageal cancer in rats. Nutr. Cancer 2011, 63, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.; Riedel, S.; Swanevelder, S.; Swart, P.; Joubert, E.; Gelderblom, W. The role of polyphenols in the modulation of skin cell viability by Aspalathus linearis and Cyclopia spp. herbal tea extracts in vitro. J. Pharm. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.; Swanevelder, S.; Swart, P.; Joubert, E.; Gelderblom, W. Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker. Molecules 2016, 21, 1323. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Hornung, B.; Lecane, P.; Jones, D.P.; Knox, S.J. Rotenone-induced G2/M cell cycle arrest and apoptosis in a human B lymphoma cell line PW. Biochem. Biophys. Res. Commun. 2001, 289, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Dyason, J.C.; Freeman, R.; Dong, L.F.; Prochazka, L.; Wang, X.F.; Scheffler, I.; Ralph, S.J. Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II. Bioenerg. Biomembr. 2007, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.O.; Kim, H.Y.; Lim, J.J.; Seo, Y.H.; Yoon, G. Mitochondrial dysfunction by complex II inhibition delays overall cell cycle progression via reactive oxygen species production. J. Cell Biochem. 2008, 104, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Tsuneoka, M.K.; Okamoto, T.K.; Tanaka, Y. Dysfunction of mitochondrial ATP production as a target for personalized cancer therapy. Curr. Pharm. Pers. Med. 2009, 7, 27–39. [Google Scholar] [CrossRef]
- Hsu, S.; Lewis, J.; Singh, B.; Schoenlein, P.; Osaki, T.; Athar, M.; Porter, A.G.; Schuster, G. Green tea polyphenol targets the mitochondria in tumor cells inducing caspase 3-dependent apoptosis. Anticancer Res. 2003, 23, 1533–1539. [Google Scholar] [PubMed]
- Hsuuw, Y.D.; Chan, W.H. Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann. N. Y. Acad. Sci. 2007, 1095, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Lou, Y.R.; Xie, J.G.; Peng, Q.Y.; Liao, J.; Yang, C.S.; Huang, M.T.; Conney, A.H. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Lou, Y.R.; Liao, J.; Xie, J.G.; Peng, Q.Y.; Yang, C.S.; Conney, A.H. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis 2005, 26, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Aziz, N.H.; Mukhtar, H.; Ahmad, N. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin. Cancer Res. 2003, 9, 3176–3182. [Google Scholar] [PubMed]
- Han, W.; Ming, M.; He, Y. Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J. Biol. Chem. 2011, 286, 22825–22832. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Anti-proliferative activity of flavonoids on several cancer cell lines. Biosci. Biotechnol. Biochem. 1999, 63, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chien, Y.S.; Chiu, T.H.; Huang, W.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012, 28, 1883–1888. [Google Scholar] [PubMed]
- Yadav, K.N.; Shukla, P.; Omer, A.; Singh, K.R. In silico approach to uncover the anti-cancerous activity of certain phyto-compounds. Gene Ther. Mol. Biol. 2013, 15, 147–158. [Google Scholar]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.C.; Ding, Y.; Green, I.R.; Gelderblom, W.C. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; de Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers (Basel) 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Veluri, R.; Kaur, M.; Chou, S.C.; Thompson, J.A.; Agarwal, R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2–3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic cell death of DU145 human prostate carcinoma cells. Carcinogenesis 2007, 28, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Howell, A.B.; Baird, M. Cranberry proanthocyanidins induce apoptosis and inhibit acid-induced proliferation of human esophageal adenocarcinoma cells. J. Agric. Food Chem. 2008, 56, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

| Cell Type | Extract | Green Tea and Herbal Teas (mg/mL) | |||
|---|---|---|---|---|---|
| C. sinensis | A. linearis | C. intermedia | C. subternata | ||
| Premalignant cells | MeOH | 0.035 ± 0.003 cA * | 0.037 ± 0.005 cA | 0.350 ± 0.077 aA | 0.190 ± 0.019 bA |
| Aq | 0.045 ± 0.007 cB * | 0.068 ± 0.011 cB | 0.202 ± 0.037 aB | 0.164 ± 0.039 bA | |
| Normal cells | MeOH | 0.063 ± 0.009 cA | 0.058 ± 0.014 cA | 0.151 ± 0.013 bA | 0.200±0.025 aA |
| Aq | 0.154 ± 0.021 bB | 0.208 ± 0.038 aB | 0.091 ± 0.022 cB | 0.098 ± 0.019 cB | |
| Cancer cells | MeOH | 0.035 ± 0.012 cA | 0.016 ± 0.003 dA * | 0.150 ± 0.046 bA | 0.223 ± 0.030 aA |
| Aq | 0.124 ± 0.025 aB | 0.048 ± 0.022 bB | 0.143 ± 0.037 aB | 0.158 ± 0.039 aB | |
| Tea/Herbal Tea | Cell Type | Units | Methanol Extract | Aqueous Extract | ||||
|---|---|---|---|---|---|---|---|---|
| C. sinensis | Premalignant cells | Extract (mg/mL) | 0.080 | 0.050 | 0.035 | 0.170 | 0.114 | 0.038 |
| Caspase-3_Fold | 5.75 ± 1.26 a | 3.54 ± 1.00 a | 1.14 ± 0.22 c,* | 6.88 ± 1.65 a | 4.94 ± 2.43 a,b | 1.57 ± 0.76 c,* | ||
| % ATP production | 36.12 ± 6.90 c | 49.08 ± 12.55a | 79.19 ± 7.79a | 47.49 ± 5.88 c | 67.63 ± 3.81b | 83.67 ± 8.18 a | ||
| Normal cells | Extract (mg/mL) | 0.230 | 0.154 | 0.051 | 0.340 | 0.228 | 0.075 | |
| Caspase-3_Fold | 4.33 ± 0.68 a | 1.98 ± 0.36 b | 1.22 ± 0.18 c,* | 5.58 ± 2.01 a | 3.20 ± 1.47 b | 1.07 ± 0.15 c,* | ||
| % ATP production | 28.58 ± 4.22 c | 50.11 ± 1.50 b | 82.37 ± 5.04 a | 42.69 ± 14.23 c | 60.64 ± 15.10 b | 95.57 ± 26.22 a | ||
| Cancer cells | Extract (mg/mL) | 0.210 | 0.141 | 0.047 | 0.410 | 0.275 | 0.091 | |
| Caspase-3_Fold | 2.13 ± 0.25 a | 1.79 ± 0.20 a | 1.26 ± 0.37 b,* | 1.55 ± 0.22 a | 1.35 ± 0.12 a | 1.13 ± 0.17 b,* | ||
| % ATP production | 62.27 ± 8.70 c | 75.41 ± 7.10 b | 96.70 ± 8.59 a | 58.92 ± 4.58 c | 73.75 ± 6.09 b | 88.35 ± 7.24 a | ||
| A. linearis | Premalignant cells | Extract (mg/mL) | 0.130 | 0.087 | 0.029 | 0.140 | 0.094 | 0.031 |
| Caspase-3_Fold | 4.67 ± 0.63 a, | 3.06 ± 0.28 a | 1.32 ± 0.40 b,* | 4.89 ± 0.78 a | 2.33 ± 0.59 b | 1.24 ± 0.25 c,* | ||
| % ATP production | 41.74 ± 5.09 c | 68.12 ± 3.27 b | 83.04 ± 4.51 a | 50.41 ± 5.35 b | 69.76 ± 7.86 b | 76.36 ± 6.61 a | ||
| Normal cells | Extract (mg/mL) | 0.260 | 0.174 | 0.058 | 0.290 | 0.194 | 0.060 | |
| Caspase-3_Fold | 2.91 ± 0.29 a | 2.46 ± 0.30 a | 1.57 ± 0.21 b,* | 1.80 ± 0.22 a | 1.71 ± 0.33 a | 1.58 ± 0.44 a,* | ||
| % ATP production | 38.68±5.91 b | 54.29 ± 9.58 b | 89.76 ± 10.73 a | 41.09 ± 6.74 b | 54.11 ± 6.55 b | 78.65 ± 15.47 a | ||
| Cancer cells | Extract (mg/mL) | 0.310 | 0.163 | 0.016 | 0.260 | 0.154 | 0.048 | |
| Caspase-3_Fold | 1.98 ± 0.17 a | 1.56 ± 0.19 b | 1.30 ± 0.24 b,* | 2.22 ± 0.25 a | 1.86 ± 0.26 a,b | 1.46 ± 0.39 b,* | ||
| % ATP production | 54.53 ± 6.51 c | 72.00 ± 5.23 b | 90.60 ± 15.84 a | 51.72 ± 2.39 c | 63.19 ± 5.56 b | 87.05 ± 6.65 a | ||
| Herbal tea | Cell Type | Units | Methanol Extract | Aqueous Extract | ||||
|---|---|---|---|---|---|---|---|---|
| C. intermedia | Premalignant cells | Extract (mg/mL) | 0.720 | 0.360 | 0.180 | 0.520 | 0.260 | 0.130 |
| Caspase-3 fold | 1.12 ± 0.34 a,* | 1.34 ± 0.29 b,* | 1.17 ± 0.25 a,* | 3.40 ± 0.95 a | 1.81 ± 0.26 b | 1.32 ± 0.18 c,* | ||
| % ATP production | 60.05 ± 12.31 b | 72.21 ± 11.72 b | 94.66 ± 4.73 a | 39.08 ± 2.50 c | 60.23 ± 8.09 b | 78.90 ± 10.09 a | ||
| Normal cells | Extract (mg/mL) | 1.370 | 0.760 | 0.150 | 0.500 | 0.295 | 0.091 | |
| Caspase-3 fold | 1.09 ± 0.11 a,* | 1.10 ± 0.18 a,* | 0.98 ± 0.26 a,* | 1.86 ± 0.61 a | 1.42 ± 0.31 a,b | 1.16 ± 0.26 b | ||
| % ATP production | 39.33 ± 4.46 c | 61.95 ± 11.40 b | 89.50 ± 20.60 a | 38.13 ± 12.42 b | 54.03 ± 11.80 b | 80.35 ± 20.17 a | ||
| Cancer cells | Extract (mg/mL) | 1.290 | 0.84 | 0.38 | 0.440 | 0.291 | 0.143 | |
| Caspase-3 fold | 0.65 ± 0.17 c | 0.94 ± 0.14 b,* | 1.40 ± 0.23 a | 1.69 ± 0.32 a | 1.51 ± 0.13 a | 1.32 ± 0.20 b,* | ||
| % ATP production | 49.57 ± 5.00 c | 66.6 ± 7.97 b | 95.48 ± 9.11 a | 48.65 ± 8.56 b | 63.32 ± 8.60 b | 92.17 ± 8.98 a | ||
| C. subternata | Premalignant cells | Extract (mg/mL) | 0.468 | 0.234 | 0.117 | 0.417 | 0.209 | 0.104 |
| Caspase-3 fold | 1.73 ± 0.20 a | 1.46 ± 0.12 a | 1.10 ± 0.25 b,* | 3.27 ± 1.00 a | 2.03 ± 0.44 b | 1.45 ± 0.22 c,* | ||
| % ATP production | 52.72 ± 10.24 c | 71.09 ± 7.65 b | 87.17 ± 9.40 a | 50.33 ± 7.21 b | 61.50 ± 8.53 b | 75.80 ± 6.51 a | ||
| Normal cells | Extract (mg/mL) | 1.080 | 0.640 | 0.200 | 0.370 | 0.230 | 0.098 | |
| Caspase-3 fold | 1.25 ± 0.27 a,* | 1.39 ± 0.37 a,* | 1.14 ± 0.16 a,* | 2.11 ± 0.61 a | 1.77 ± 0.37 a,b | 1.45 ± 0.27 a,* | ||
| % ATP production | 40.17 ± 6.89 c | 64.71 ± 5.99 b | 84.98 ± 8.28 a | 49.96 ± 6.09 c | 63.28 ± 7.45 b | 80.42 ± 9.88 a | ||
| Cancer cells | Extract (mg/mL) | 1.140 | 0.680 | 0.223 | 0.430 | 0.294 | 0.158 | |
| Caspase-3 fold increase | 0.97 ± 0.26 a,* | 1.04 ± 0.12 a,* | 1.15 ± 0.06 a,* | 1.45 ± 0.33 a,* | 1.55 ± 0.35 a,* | 1.28 ± 0.23 a,* | ||
| % ATP production | 36.09 ± 1.31 c | 60.68 ± 3.46 b | 76.79 ± 0.70 a | 39.68 ± 2.28 c | 66.38 ± 1.77 b | 82.00 ± 8.73 a | ||
| Tea/Herbal Tea | Methanol Extracts | Aqueous Extracts | ||||
|---|---|---|---|---|---|---|
| Premalignant Cells | Normal Cells | Cancer Cells | Premalignant Cells | Normal Cells | Cancer Cells | |
| C. sinensis | −0.881 | −0.888 | −0.800 | −0.742 | −0.878 | −0.672 |
| (<0.0001) | (<0.0001) | (<0.0001) | (<0.0001) | (<0.0001) | (<0.0001) | |
| A. linearis | −0.881 | −0.802 | −0.769 | −0.745 | −0.547 | −0.724 |
| (<0.0001) | (<0.0001) | (<0.0001) | (=0.0001) | (=0.0002) | (<0.0001) | |
| C. subternata | −0.841 | −0.588 | - | −0.783 | −0.864 | −0.554 |
| (<0.0001) | (=0.0004) | - | (<0.0001) | (<0.0001) | (=0.0027) | |
| C. intermedia | −0.527 | - | 0.479 | −0.819 | −0.699 | −0.599 |
| (=0.0005) | - | (=0.0015) | (<0.0001) | (<0.0001) | (<0.0001) | |
| Tea/Herbal Extracts | Polyphenols * | Concentration * (µg/mg Extract) | TP/FLAVA Ratio | ||
|---|---|---|---|---|---|
| Methanol | Aqueous | Methanol | Aqueous | ||
| C. sinensis | TP | 256.5 ± 36.9 A | 161.0 ± 20.8 B | 1.94 | 2.07 |
| FLAVA | 132.3 ± 3.4 A | 77.6 ± 1.5 B | |||
| EGCG | 111.9 ± 3.0 A | 46.1 ± 1.5 B | |||
| Total flavanols | 200.8 ± 6.2 A | 100.0 ± 2.8 B | |||
| A. linearis | TP | 350.7 ± 35.0 A | 250.5 ± 16.4 B | 12.92 | 13.92 |
| FLAVA | 27.1 ± 0.8 A | 18.0 ± 0.6 B | |||
| DHC | 151.8 ± 1.7 A | 100.6 ± 1.8 B | |||
| Total_ mono | 209.1 ± 1.8 A | 138.1 ± 3.6 B | |||
| C. intermedia | TP | 172.1 ± 4.1 A | 164.5 ± 11.3 B | 15.23 | 9.19 |
| FLAVA | 11.3 ± 0.9 B | 17.9 ± 0.9 A | |||
| Xanthones | 87.8 ± 2.9 A | 54.0 ± 0.8 B | |||
| Hesperidin | 88.8 ± 11.6 A | 7.3 ± 0.6 B | |||
| Total_ mono | 186.2 ± 13.0 A | 64.0 ± 0.6 B | |||
| C. subternata | TP | 220.5 ± 14.5 A | 175.0 ± 24.1 B | 16.96 | 7.64 |
| FLAVA | 13.0 ± 0.6 B | 22.9 ± 0.9 A | |||
| Xanthones | 78.5 ± 1.3 A | 30.7 ± 4.4 B | |||
| Hesperidin | 63.1 ± 8.6 A | 8.0 ± 0.2 B | |||
| Total_mono | 174.4 ± 9.0 A | 51.3 ± 4.5 B | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magcwebeba, T.U.; Swart, P.; Swanevelder, S.; Joubert, E.; Gelderblom, W.C.A. In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells. Molecules 2016, 21, 1622. https://doi.org/10.3390/molecules21121622
Magcwebeba TU, Swart P, Swanevelder S, Joubert E, Gelderblom WCA. In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells. Molecules. 2016; 21(12):1622. https://doi.org/10.3390/molecules21121622
Chicago/Turabian StyleMagcwebeba, Tandeka U., Pieter Swart, Sonja Swanevelder, Elizabeth Joubert, and Wentzel C. A. Gelderblom. 2016. "In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells" Molecules 21, no. 12: 1622. https://doi.org/10.3390/molecules21121622
APA StyleMagcwebeba, T. U., Swart, P., Swanevelder, S., Joubert, E., & Gelderblom, W. C. A. (2016). In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells. Molecules, 21(12), 1622. https://doi.org/10.3390/molecules21121622






