The Protective Effect of Selenium on Chronic Zearalenone-Induced Reproductive System Damage in Male Mice
Abstract
:1. Introduction
2. Results
2.1. Organ Indexes of Epididymis and Testis
2.2. Serum Level of Testosterone
2.3. Sperm Morphology and Deformity Rate
2.4. Sperm Concentration and Motility Parameters
2.5. Testis Antioxidant Parameters
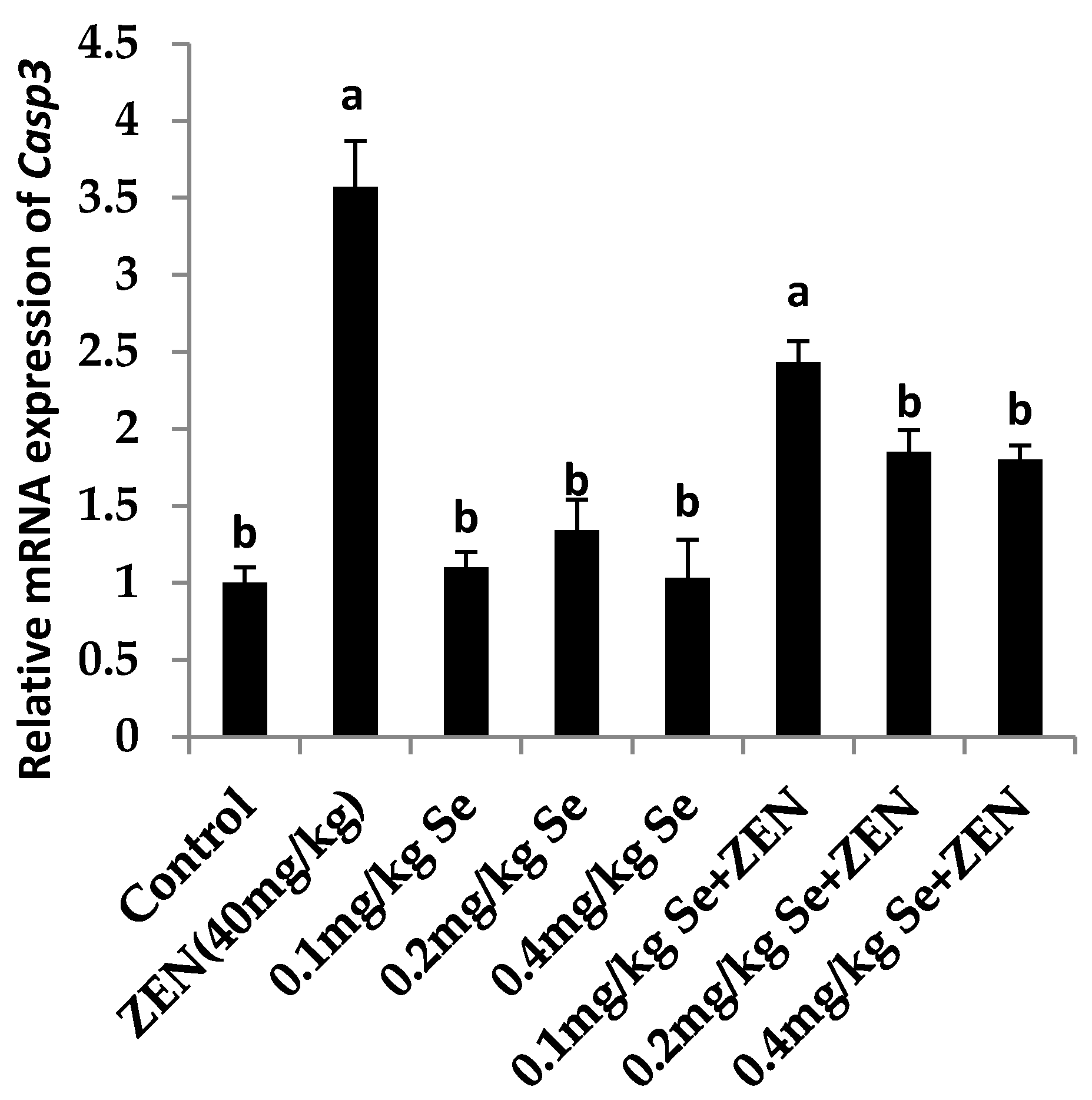
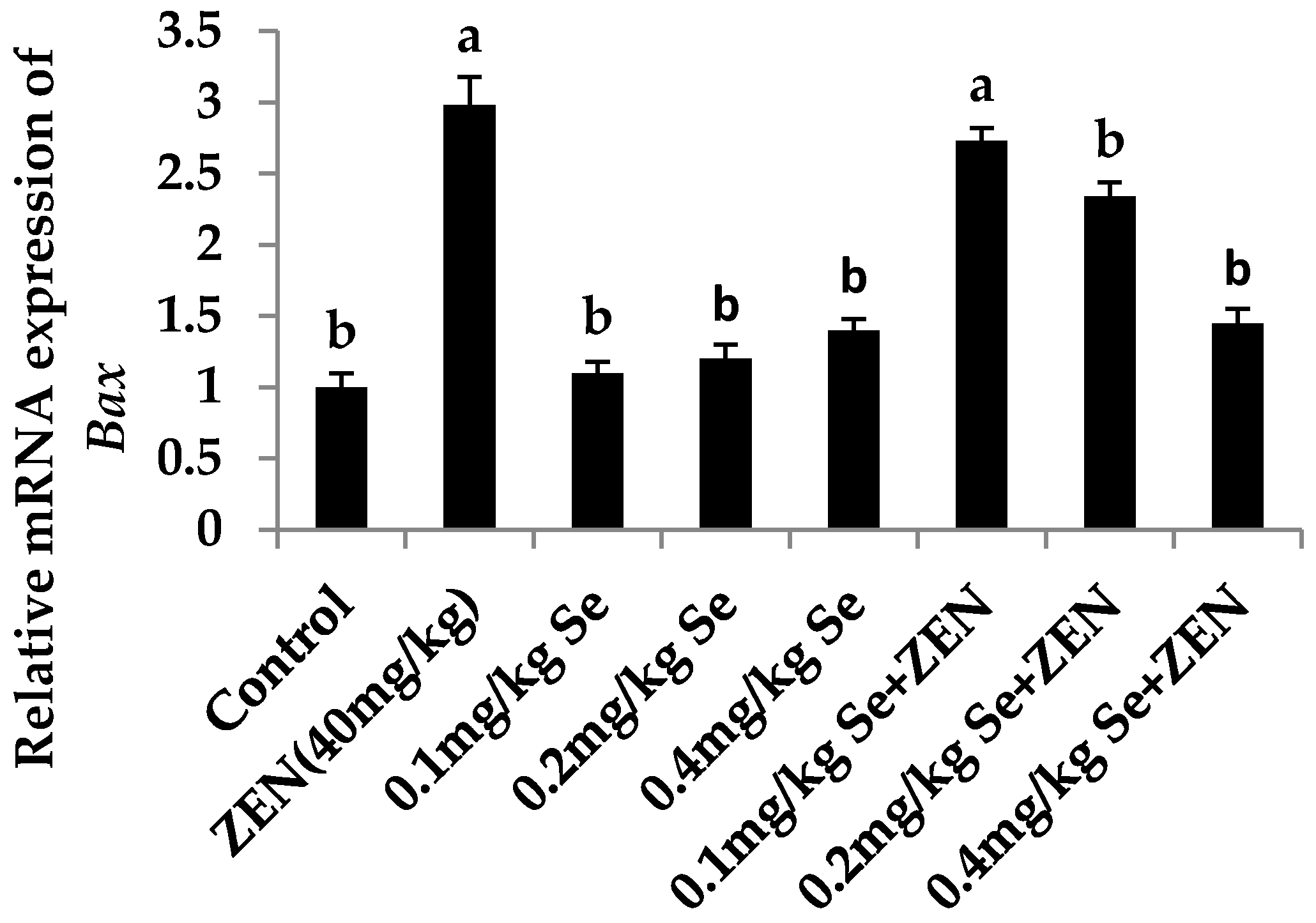
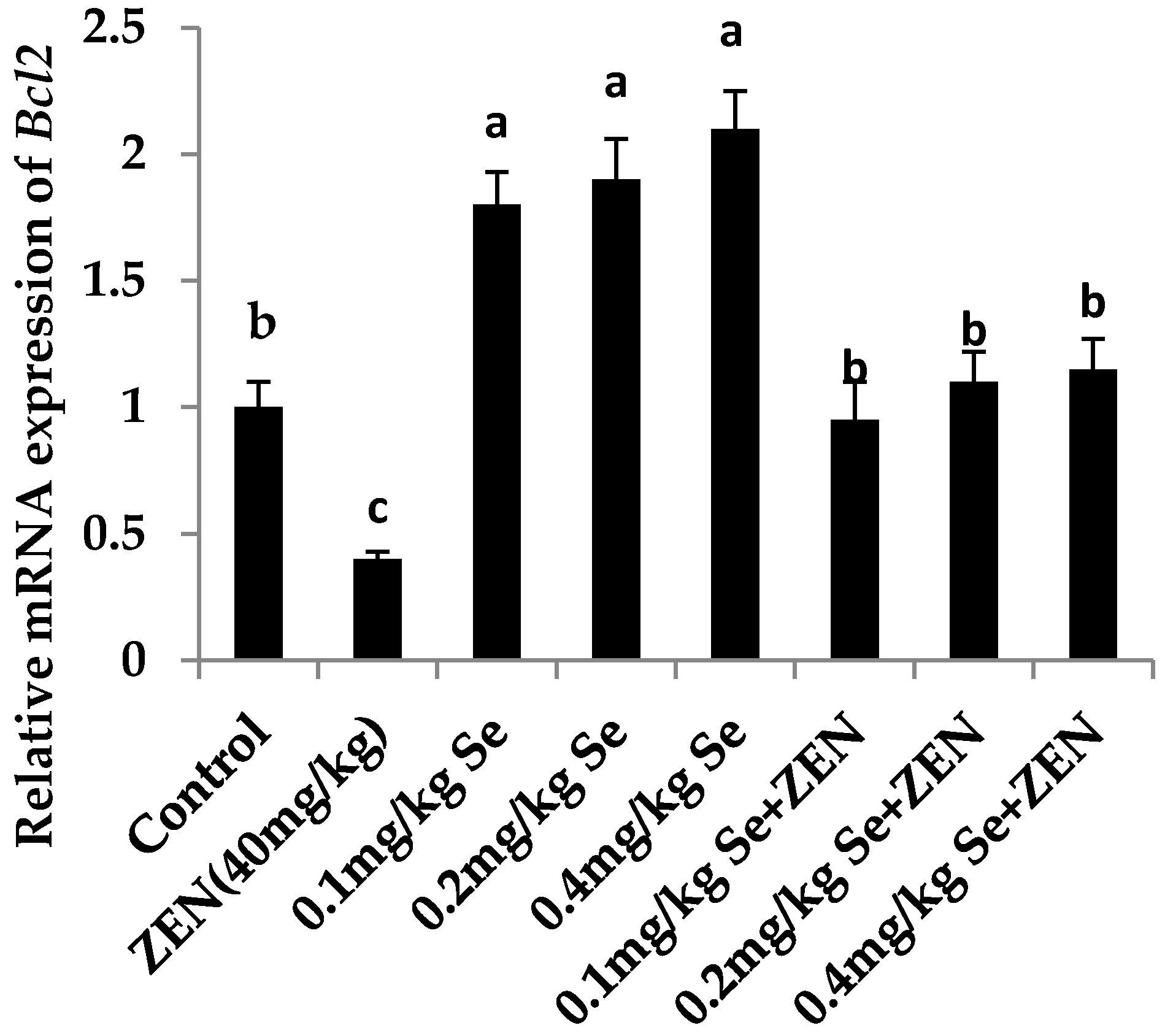
2.6. Effect on the mRNA Expression of Bax, Casp3, and Bcl2
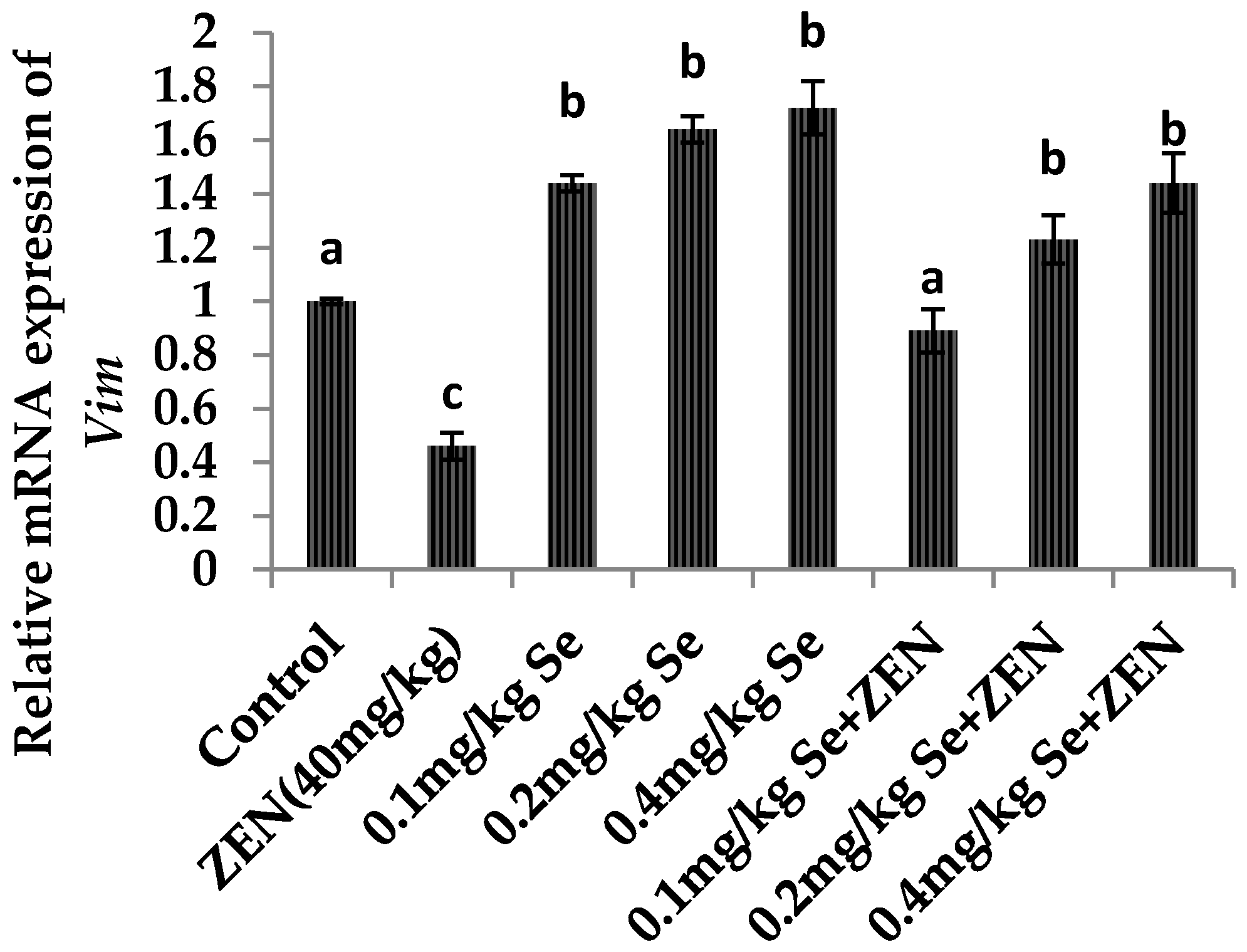
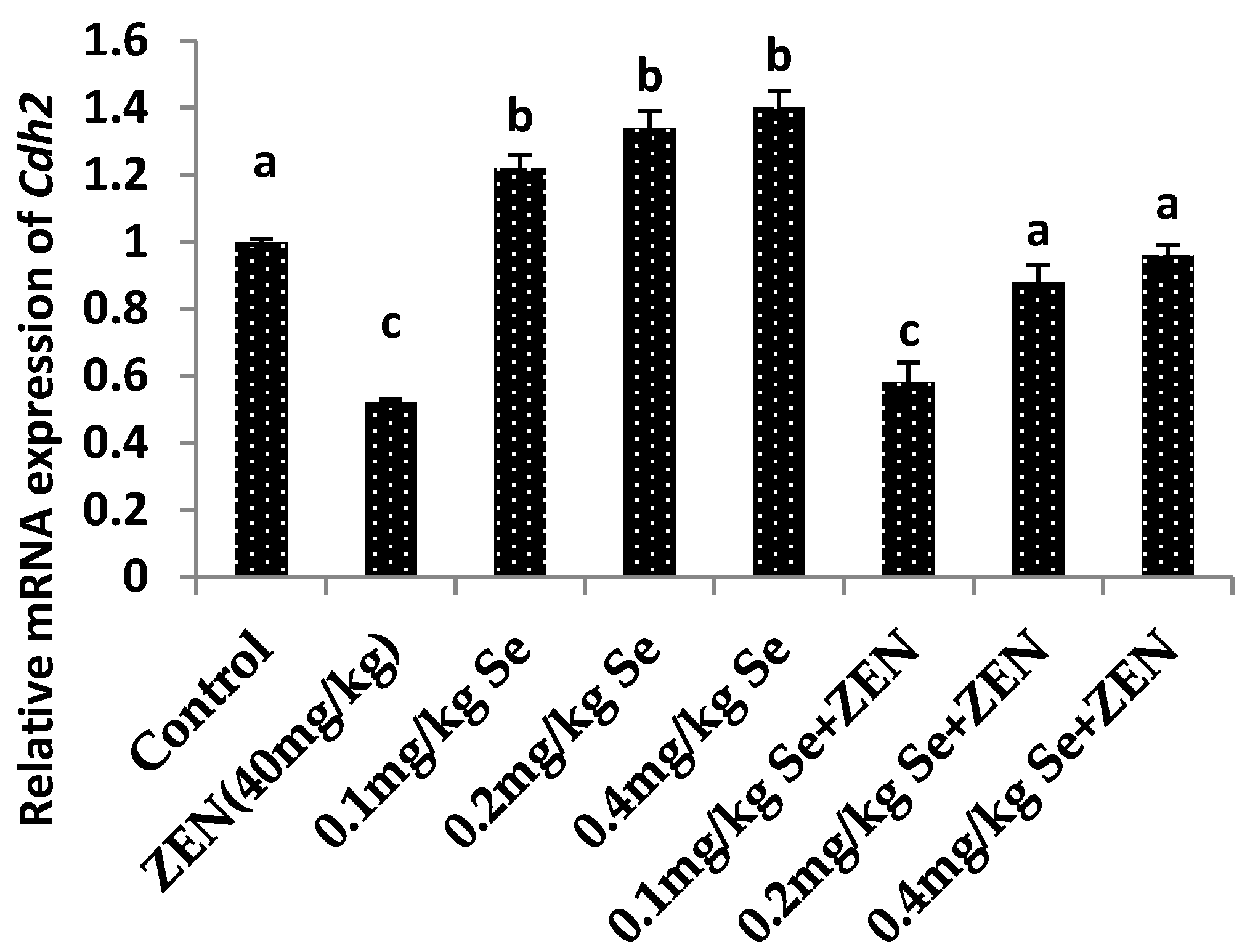
2.7. Effect on the mRNA Expression of Vim and Cdh2
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Chemicals
4.3. Experimental Design and Treatment
4.4. Parameters
4.4.1. Epididymis, and Testis, Indexes
4.4.2. Serum Testosterone Levels in Mice
4.4.3. Sperm Deformity Rate
4.4.4. Sperm Concentration and Motility
4.4.5. Antioxidant
4.5. Gene Expression
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hestbjerg, H.; Nielsen, K.F.; Thrane, U.; Elmholt, S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: An ecological interpretation. J. Agric. Food. Chem. 2002, 50, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control. 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Mac Donald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Woźny, M.; Brzuzan, P.; Zielonka, Ł.; Gajęcki, M. Expression of CYPscc and 3β-HSD mRNA in bitches ovary after long-term exposure to zearalenone. Bull. Vet. Inst. Pulawy 2011, 55, 777–780. [Google Scholar]
- Winkler, J.; Kersten, S.; Meyer, U.; Engelhardt, U.; Dänicke, S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food. Chem. Toxicol. 2014, 65, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Gajęcki, M. Zearalenone: Undesirable substances in feed. Pol. J. Vet. Sci. 2002, 5, 117–122. [Google Scholar] [PubMed]
- Ben Salah-Abbes, J.; Abbes, S.; Houas, Z.; Abdel-Wahhab, M.A.; Oueslati, R. Zearalenone induces immunotoxicity in mice: possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. 2008, 60, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Conkova, E.; Laciakova, A.; Pastorova, B.; Seidel, H.; Kovac, G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001, 121, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.; Raspantini, L.E.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins (Basel) 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lioi, M.B.; Santoro, A.; Barbieri, R.; Salzano, S.; Ursini, M.V. Ochratoxin and zearalenone: A comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res. 2004, 557, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.E.; Bacha, H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol. In Vitro 2004, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food. Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Hu, J.; Guo, B.P.; Niu, Y.R.; Xiao, C.; Xu, Y.X. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone-treated rat sertoli cells. Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, M.P. Protective role of dietary-supplemented selenium and vitamin E in heat-induced apoptosis and oxidative stress in mice testes. Andrologia 2015, 47, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yao, X.; Shi, L.; Ren, Y.; Zhao, H. Effects of dietary selenium on apoptosis of germ cells in the testis during spermatogenesis in roosters. Theriogenology 2015, 84, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kara, Ö.; Sari, E.; Akşit, H.; Yay, A.; Akşit, D.; Dönmez, M.I. Effects of selenium on ischaemia-reperfusion injury in a rat testis model. Andrologia 2016. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, F.; Liang, N.; Wang, C.; Peng, X.; Fang, J.; Cui, H.; Jameel Mughal, M.; Lai, W. Effect of selenium supplementation on apoptosis and cell cycle blockage of renal cells in broilers fed a diet Containing Aflatoxin B1. Biol. Trace Elem. Res. 2015, 68, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Zhang, W.; Zhang, Y.; Li, P.; Guo, Y.; Wang, Y.; Chen, X.; He, J. The influence of selenium yeast on hematological, biochemical and reproductive hormone level changes in Kunming Mice following acute exposure to zearalenone. Biol. Trace Elem. Res. 2016, 174, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Xue, K.; Gu, G.; Fan, W.; Xu, Y. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS ONE 2015, 10, e0120775. [Google Scholar] [CrossRef] [PubMed]
- Bekheet, S.H.M. Cadmium chloride rapidly alters both BTB tight junction proteins and germ cells in young rat testes. Egypt. Acad. J. Biol. Sci. 2010, 2, 59–74. [Google Scholar]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood-testis barrier and spermatogenesis: lessons from genetically-modified mice. Asian J. Androl. 2014, 16, 572–580. [Google Scholar] [PubMed]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. Sci. USA 2010, 107, 17998–18003. [Google Scholar] [CrossRef] [PubMed]
- Carette, D.; Perrard, M.H.; Prisant, N.; Gilleron, J.; Pointis, G.; Segretain, D.; Durand, P. Hexavalent chromium at low concentration alters Sertoli cell barrier and connexin 43 gap junction but not claudin-11 and N-cadherin in the rat seminiferous tubule culture model. Toxicol. Appl. Pharmacol. 2013, 268, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Iliadou, P.K.; Tsametis, C.; Kaprara, A.; Papadimas, I.; Goulis, D.G. The Sertoli cell: Novel clinical potentiality. Hormones (Athens) 2015, 14, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Wang, Y.; Cui, S. Toxic effects of zearalenone and α-zearalenol on the regulation of steroidogenesis and testosterone production in mouse Leydig cells. Toxicol. In Vitro 2007, 21, 558–565. [Google Scholar] [CrossRef] [PubMed]
- El Golli Bennour, E.; Bouaziz, C.; Ladjimi, M.; Renaud, F.; Bacha, H. Comparative mechanisms of zearalenone and ochratoxin A toxicities on cultured HepG2 cells: Is oxidative stress a common process? Environ. Toxicol. 2009, 24, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; Lopez Ade, C.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef] [PubMed]
- El Golli-Bennour, E.; Bacha, H. Hsp70 expression as biomarkers of oxidative stress: Mycotoxins’ exploration. Toxicology 2011, 287, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah-Abbès, J.; Abbès, S.; Abdel-Wahhab, M.A.; Oueslati, R. Raphanus sativus extract protects against Zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon 2009, 53, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Addition of Selenium Improves Immunomodulative Effects of Glucan. N. Am. J. Med. Sci. 2016, 8, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Hu, J.; Song, S.; Huang, D.; Xu, H.; Qian, G.; Gan, F.; Huang, K. Selenium Alleviates Aflatoxin B1-Induced Immune Toxicity through Improving Glutathione Peroxidase 1 and Selenoprotein S Expression in Primary Porcine Splenocytes. J. Agric. Food. Chem. 2016, 64, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.C.; Matulka, R.A.; Power, R. Acute and subchronic toxicity studies on Sel-Plex, a standardized, registered highselenium yeast. Int. J. Toxicol. 2006, 25, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Kurzepa, J.; Lewandowska, A.; Żelazowska, R.; Musik, I. Could selenium administration alleviate the disturbances of blood parameters caused by lithium administration in rats? Biol. Trace Elem. Res. 2014, 158, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, B.; Cheng, Y.; Lin, H. Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol. Trace Elem. Res. 2015, 167, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.C.; Blaschuk, O.W.; Millette, C.F. N-cadherin mediates Sertoli cell- spermatogenic cell adhesion. Dev. Dyn. 1993, 197, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G. Cyr. Connexins and pannexins Coordinating cellular communication in the testis and epididymis. Spermatogenesis 2011, 1, 325–338. [Google Scholar]
- Zheng, W.; Pan, S.; Wang, G.; Wang, Y.J.; Liu, Q.; Gu, J.; Yuan, Y.; Liu, X.Z.; Liu, Z.P.; Bian, J.C. Zearalenone impairs the male reproductive system functions via inducing structural and functional alterations of sertoli cells. Environ. Toxicol. Pharmacol. 2016, 42, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Taskin, E.; Dursun, N. The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol. Trace Elem. Res. 2012, 147, 165–171. [Google Scholar] [CrossRef] [PubMed]
- El-Sharaky, A.; Newairy, A.; Badreldeen, M.; Eweda, S.; Sheweita, S. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Wang, F.; Peng, X.; Fang, J.; Cui, H.; Chen, Z.; Lai, W.; Zhou, Y.; Geng, Y. Effect of Sodium Selenite on Pathological Changes and Renal Functions in Broilers Fed a Diet Containing Aflatoxin B1. Int. J. Environ. Res. Public Health 2015, 12, 11196–11208. [Google Scholar] [CrossRef] [PubMed]
- Simsek, N.; Koc, A.; Karadeniz, A.; Yildirim, M.E.; Celik, H.T.; Sari, E.; Kara, A. Ameliorative effect of selenium in cisplatin-induced testicular damage in rats. Acta Histochem. 2016, 118, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Saygin, M.; Caliskan, S.; Ozguner, M.F.; Gumral, N.; Comlekci, S.; Karahan, N. Impact of l-carnitine and Selenium Treatment on Testicular Apoptosis in Rats Exposed to 2.45 GHz Microwave Energy. West. Indian Med. J. 2015, 64, 55–61. [Google Scholar] [PubMed]
- Sakr, S.A.; Mahran, H.A.; Nofal, A.E. Effect of selenium on carbimazole-induced testicular damage and oxidative stress in albino rats. J. Trace Elem. Med. Biol. 2011, 25, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Funck, V.R.; Filho, C.B.; Del’Fabbro, L.; de Gomes, M.G.; Donato, F.; Royes, L.F.; Oliveira, M.S.; Jesse, C.R.; Furian, A.F. Lycopene protects against acute zearalenone-induced oxidative, endocrine, inflammatory and reproductive damages in male mice. Chem. Biol. Interact. 2015, 230, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Filho, C.B.; Del’Fabbro, L.; Roman, S.S.; Royes, L.F.; Fighera, M.R. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp. Toxicol. Pathol. 2014, 66, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
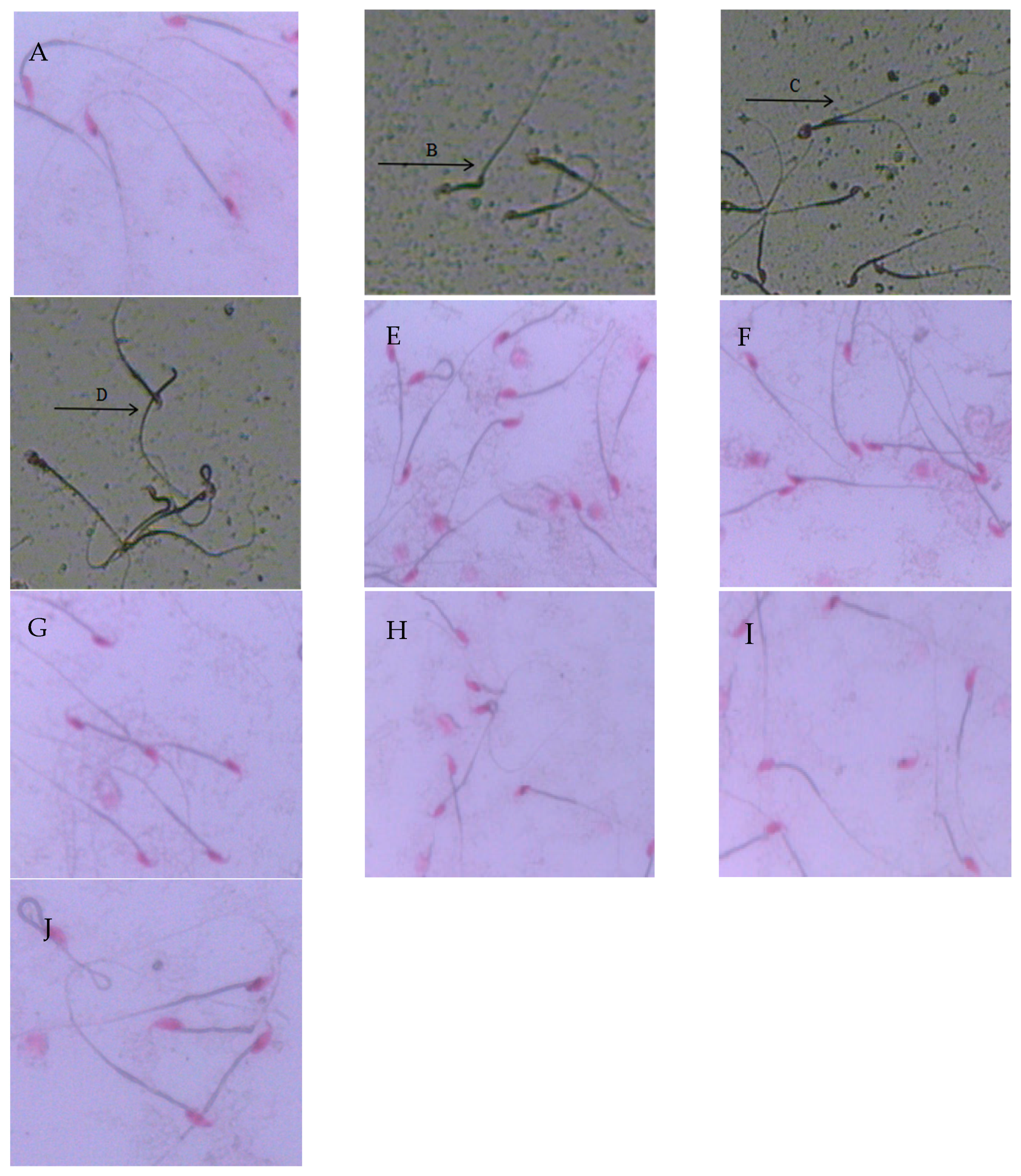
| Group | Organ Indexes of Epididymis (%) | Organ Indexes of Testis (%) |
|---|---|---|
| Control | 0.108 ± 0.006 b | 0.393 ± 0.012 b |
| ZEN (40 mg/kg) | 0.073 ± 0.004 a | 0.305 ± 0.006 a |
| 0.1 mg/kg Se | 0.112 ± 0.003 b | 0.418 ± 0.009 b |
| 0.2 mg/kg Se | 0.120 ± 0.008 c | 0.433 ± 0.010 c |
| 0.4 mg/kg Se | 0.125 ± 0.005 c | 0.440 ± 0.007 c |
| 0.1 mg/kg Se + ZEN | 0.093 ± 0.006 b | 0.410 ± 0.005 b |
| 0.2 mg/kg Se + ZEN | 0.095 ± 0.005 b | 0.416 ± 0.006 b |
| 0.4 mg/kg Se + ZEN | 0.097 ± 0.008 b | 0.420 ± 0.008 b |
| Group | Serum Level of Testosterone (ng/mL) |
|---|---|
| Control | 7.443 ± 1.064 c |
| ZEN (40 mg/kg) | 0.153 ± 0.008 a |
| 0.1 mg/kg Se | 9.973 ± 1.107 b |
| 0.2 mg/kg Se | 10.404 ± 1.139 b |
| 0.4 mg/kg Se | 11.967 ± 1.007 b |
| 0.1 mg/kg Se + ZEN | 0.684 ± 0.011 b |
| 0.2 mg/kg Se + ZEN | 0.933 ± 0.005 b |
| 0.4 mg/kg Se + ZEN | 1.475 ± 0.007 b |
| Group | Sperm Deformity Rate (%) | Sperm Number (million/mL) | Motile (%) | Progressive (%) | VAP (μm/s) |
|---|---|---|---|---|---|
| Control | 12.22 ± 2.11 a | 21.49 ± 1.58 a | 90.41 ± 2.17 a | 15.33 ± 2.77 a | 75.42 ± 4.34 a |
| ZEN (40 mg/kg) | 24.56 ± 4.20 b | 10.06 ± 0.55 b | 38.52 ± 1.30 b | 6.78 ± 1.49 b | 48.72 ± 3.33 b |
| 0.1 mg/kg Se | 13.58 ± 3.66 a | 22.42 ± 1.80 a | 90.36 ± 1.85 a | 15.03 ± 1.74 a | 74.32 ± 2.86 a |
| 0.2 mg/kg Se | 13.23 ± 3.26 a | 21.51 ± 1.88 a | 91.54 ± 1.80 a | 15.65 ± 1.88 a | 76.73 ± 2.56 a |
| 0.4 mg/kg Se | 12.56 ± 3.41 a | 22.98 ± 1.61 a | 92.30 ± 1.63 a | 16.38 ± 1.93 a | 80.16 ± 2.21 a |
| 0.1 mg/kg Se + ZEN | 20.84 ± 3.52 b | 12.33 ± 0.91 b | 81.56 ± 1.39 c | 6.66 ± 1.04 b | 54.33 ± 1.59 b |
| 0.2 mg/kg Se + ZEN | 20.46 ± 3.09 b | 16.06 ± 1.23 c | 82.82 ± 1.31 c | 7.28 ± 1.61 b | 70.43 ± 1.69 a |
| 0.4 mg/kg Se + ZEN | 13.45 ± 2.52 a | 20.15 ± 1.43 a | 88.36 ± 1.53 a | 14.46 ± 1.47 a | 72.40 ± 1.58 a |
| Group | MDA (nmol/mgprot) | SOD (U/mgprot) | GPx (mg/gprot) |
|---|---|---|---|
| control | 0.437 ± 0.067 b | 55.149 ± 0.788 b | 47.118 ± 3.878 b |
| ZEN (40 mg/kg) | 1.255 ± 0.039 c | 35.006 ± 0.450 c | 29.112 ± 1.80 c |
| 0.1 mg/kg Se | 0.510 ± 0.057 b | 65.429 ± 0.800 a | 55.460 ± 3.45 b |
| 0.2 mg/kg Se | 0.526 ± 0.068 b | 67.713 ± 1.088 a | 57.421 ± 3.088 b |
| 0.4 mg/kg Se | 0.683 ± 0.037 b | 68.998 ± 2.310 a | 75.460 ± 1.230 a |
| 0.1 mg/kg Se + ZEN | 1.182 ± 0.021 c | 56.630 ± 1.319 b | 29.604 ± 3.319 c |
| 0.2 mg/kg Se + ZEN | 0.981 ± 0.038 c | 59.006 ± 1.031 b | 32.421 ± 3.301 b |
| 0.4 mg/kg Se + ZEN | 0.710 ± 0.020 b | 60.050 ± 1.43 b | 45.625 ± 3.432 b |
| Gene | Primer | Primer Sequences (5’-3’) | Product Size/bp | Accession No. |
|---|---|---|---|---|
| Casp3 | Forward | CTGACTGGAAAGCCGAAACTC | 189 bp | NM_009810.2 |
| Reverse | CGACCCGTCCTTTGAATTTCT | |||
| Bax | Forward | CAGGATGCGTCCACCAAGAA | 197 bp | NM_007527.3 |
| Reverse | GCAAAGTAGAAGAGGGCAACCAC | |||
| Bcl2 | Forward | GCTACCGTCGTCGTGACTTCGC | 147 bp | NM_177410.2 |
| Reverse | CCCCACCGAACTCAAAGAAGG | |||
| Vim | Forward | GATCAGCTCACCAACGACAA | 120 bp | NM_011701.4 |
| Reverse | GCTTTCGGCTTCCTCTCTCT | |||
| Cdh2 | Forward | AGGACCCTTTCCTCAAGAGC | 117 bp | AB008811.1 |
| Reverse | ATAATGAAGATGCCCGTTGG | |||
| Actb | Forward | CTGTCCCTGTATGCCTCTG | 221 bp | BC_138614.1 |
| Reverse | TTGATGTCACGCACGATT |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Yang, S.; Wang, Y.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.; He, J.; Gao, Z.; et al. The Protective Effect of Selenium on Chronic Zearalenone-Induced Reproductive System Damage in Male Mice. Molecules 2016, 21, 1687. https://doi.org/10.3390/molecules21121687
Long M, Yang S, Wang Y, Li P, Zhang Y, Dong S, Chen X, Guo J, He J, Gao Z, et al. The Protective Effect of Selenium on Chronic Zearalenone-Induced Reproductive System Damage in Male Mice. Molecules. 2016; 21(12):1687. https://doi.org/10.3390/molecules21121687
Chicago/Turabian StyleLong, Miao, Shuhua Yang, Yuan Wang, Peng Li, Yi Zhang, Shuang Dong, Xinliang Chen, Jiayi Guo, Jianbin He, Zenggui Gao, and et al. 2016. "The Protective Effect of Selenium on Chronic Zearalenone-Induced Reproductive System Damage in Male Mice" Molecules 21, no. 12: 1687. https://doi.org/10.3390/molecules21121687






