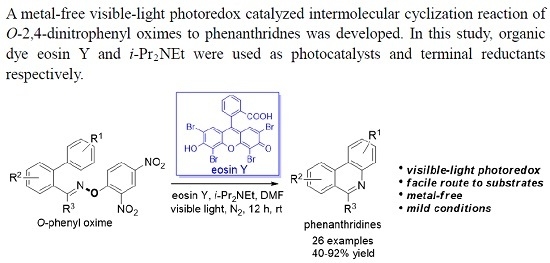
Metal-Free Photoredox Catalyzed Cyclization of O-(2,4-Dinitrophenyl)oximes to Phenanthridines
Abstract
:1. Introduction
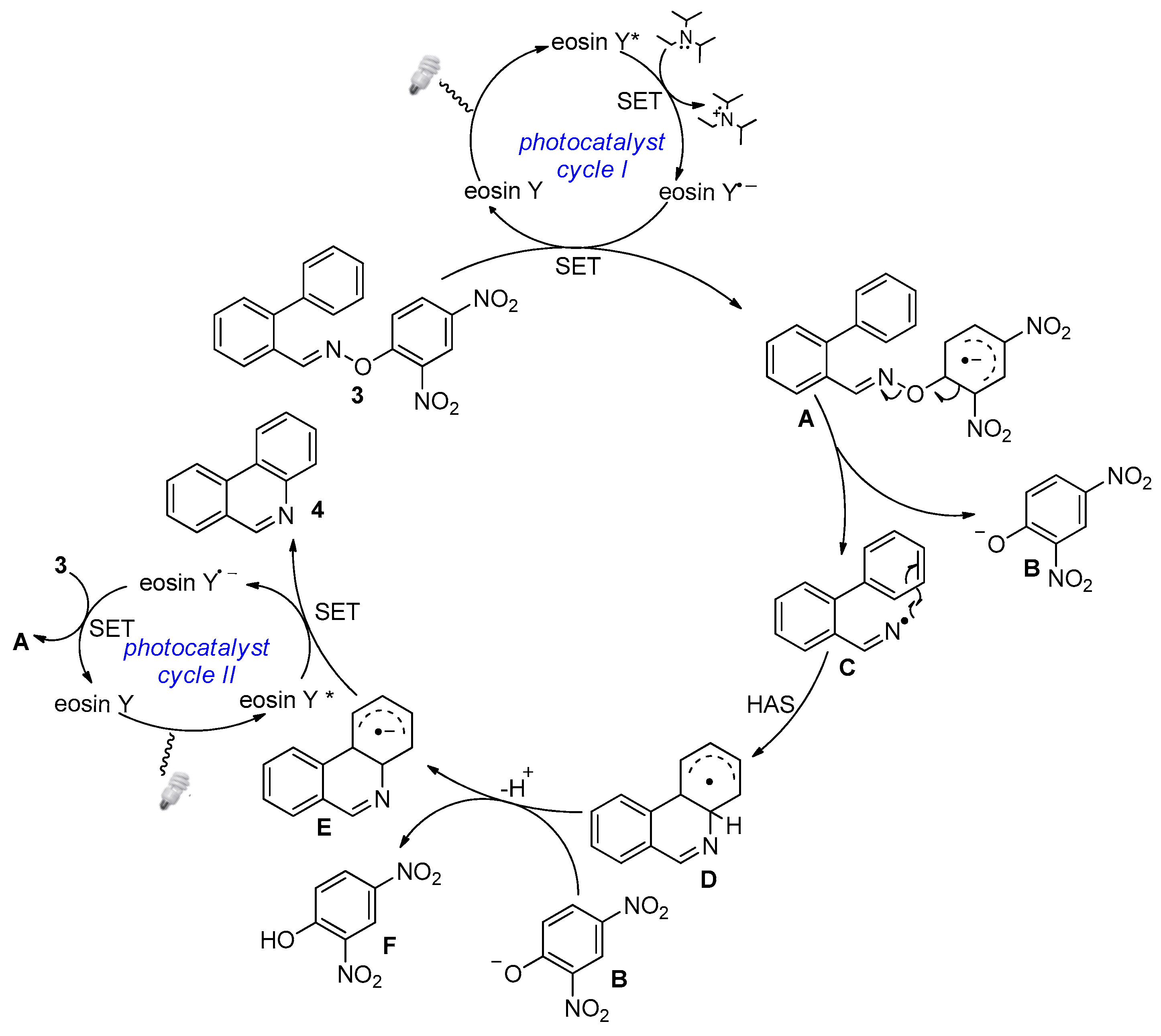
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Representative Experimental Procedure for Visible Light Promoted Synthesis of Phenanthridines 4 and 7
3.3. Physical, Analytical and Spectral Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zenk, M.H. The formation of benzophenanthridine alkaloids. Pure Appl. Chem. 1994, 66, 2023–2028. [Google Scholar] [CrossRef]
- Nakanishi, T.; Suzuki, M. Revision of the structure of fagaridine based on the comparison of UV and NMR data of synthetic compounds. J. Nat. Prod. 1998, 61, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Masuda, A.; Suwa, M.; Akiyama, Y.; Hoshino-Abe, N.; Suzuki, M. Synthesis of derivatives of NK109, 7-OH benzo[c]phenanthridine alkaloid, and evaluation of their cytotoxicities and reduction-resistant properties. Bioorg. Med. Chem. Lett. 2000, 10, 2321–2323. [Google Scholar] [CrossRef]
- Nakanishi, T.; Suzuki, M.; Mashiba, A.; Ishikawa, K.; Yokotsuka, T. Synthesis of NK109, an anticancer benzo[c]phenanthridine alkaloid. J. Org. Chem. 1998, 63, 4235–4239. [Google Scholar] [CrossRef]
- Newman, S.E.; Roll, M.J.; Harkrader, R.J. A naturally occurring compound for controlling powdery mildew of greenhouse roses. HortScience 1999, 34, 686–689. [Google Scholar]
- Seaman, A.; Woodbine, M. The antibacterial activity of phenanthridine compounds. Brit. J. Pharmacol. 1954, 3, 265–270. [Google Scholar] [CrossRef]
- Schrader, K.K.; Avolio, F.; Andolfi, A.; Cimmino, A.; Evidente, A. Ungeremine and its hemisynthesized analogues as bactericides against Flavobacterium columnare. J. Agric. Food Chem. 2013, 61, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Kellinger, M.W.; Park, G.Y.; Chong, J.; Lippard, S.J.; Wang, D. Effect of a monofunctional phenanthriplatin-DNA adduct on RNA polymerase II transcriptional fidelity and translesion synthesis. J. Am. Chem. Soc. 2013, 135, 13054–13061. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Alexander, S.M.; Lin, W.; Lippard, S.J. Effects of monofunctional platinum agents on bacterial growth: A retrospective study. J. Am. Chem. Soc. 2014, 136, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Baechler, S.A.; Fehr, M.; Habermeyer, M.; Hofmann, A.; Merz, K.; Fiebig, H.; Marko, D.; Eisenbrand, G. Synthesis, topoisomerase-targeting activity and growth inhibition of lycobetaine analogs. Bioorg. Med. Chem. 2013, 21, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhou, J.; Qing, Z.; Kang, W.; Liu, S.; Liu, W.; Xie, H.; Zeng, J. Synthesis of 5-methyl phenanthridium derivatives: A new class of human DOPA decarboxylase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.; O’Connor, N.; Vishwasrao, H.; Samaroo, D.; Kandel, E.R.; Akins, D.L.; Drain, C.M.; Turro, N. Two color RNA intercalating probe for cell imaging applications. J. Am. Chem. Soc. 2008, 130, 7182–7183. [Google Scholar] [CrossRef] [PubMed]
- Candito, D.A.; Lautens, M. Palladium-catalyzed domino direct arylation/N-arylation: Convenient synthesis of phenanthridines. Angew. Chem. Int. Ed. 2009, 48, 6713–6716. [Google Scholar] [CrossRef] [PubMed]
- Blanchot, M.; Candito, D.A.; Larnaud, F.; Lautens, M. Formal synthesis of nitidine and NK109 via palladium-catalyzed domino direct arylation/N-arylation of aryl triflates. Org. Lett. 2011, 13, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Studer, A. 6-Trifluoromethylphenanthridines through radical trifluoromethylation of isonitriles. Angew. Chem. Int. Ed. 2013, 52, 10792–10795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. 2-Trifluoromethylated indoles via radical trifluoromethylation of isonitriles. Org. Lett. 2014, 16, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. 6-Perfluoroalkylated phenanthridines via radical perfluoroalkylation of isonitriles. Org. Lett. 2014, 16, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
- Rortela-Cubillo, F.; Scott, J.S.; Walton, J.C. Microwave-assisted preparations of dihydropyrroles from alkenone O-phenyl oximes. Chem. Commun. 2007, 4041–4043. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, F.; Scanlan, E.M.; Scott, J.S.; Walton, J.C. From dioxime oxalates to dihydropyrroles and phenanthridines via iminyl radicals. Chem. Commun. 2008, 4189–4191. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, F.; Scott, J.S.; Walton, J.C. Microwave-assisted syntheses of N-heterocycles using alkenone-, alkynone- and aryl-carbonyl O-phenyl oximes: Formal synthesis of neocryptolepine. J. Org. Chem. 2008, 73, 5558–5565. [Google Scholar] [CrossRef] [PubMed]
- McBurney, R.T.; Walton, J.C. Dissociation or cyclization: Options for a triad of radicals released from oxime carbamates. J. Am. Chem. Soc. 2013, 135, 7349–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, J.C. The oxime portmanteau motif: Released heteroradicals undergo incisive EPR interrogation and deliver diverse heterocycles. Acc. Chem. Res. 2014, 47, 1406–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible-light-promoted iminyl-radical formation from acyl oximes: A unified approach to pyridines, quinolines, and phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- An, X.-D.; Yu, S. Visible-light-promoted and one-pot synthesis of phenanthridines and quinolines from aldehydes and O-acyl hydroxylamine. Org. Lett. 2015, 17, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Booth, S.G.; Essafi, S.; Dryfe, R.A.W.; Leonori, D. Visible-light-mediated generation of nitrogen-centered radicals: Metal-free hydroimination and iminohydroxylation cyclization reactions. Angew. Chem. Int. Ed. 2015, 54, 14017–14041. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Svejstrup, T.D.; Reina, D.F.; Sheikh, N.S.; Leonori, D. Visible-light-mediated synthesis of amidyl radicals: Transition-metal-free hydroamination and N-arylation reactions. J. Am. Chem. Soc. 2016, 138, 8092–8095. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zeng, J. Progresses in synthesis of benzophenanthridine alkaloids and their derivatives. Chin. J. Org. Chem. 2012, 32, 1605–1619. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Liu, W.; Liu, X.; Liu, F.; Huang, P.; Zhu, P.; Chen, J.; Shi, M.; Guo, F.; et al. Integration of transcriptome, proteome and metabolism data reveals the alkaloids biosynthesis in Macleaya cordata and Macleaya microcarpa. PLoS ONE 2013, 8, e53409. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Cheng, P.; Liu, X.-B.; Liu, Y.-S.; Zeng, J.-G.; Wang, W. Structural speculation and identification of alkaloids in Macleaya cordata fruits by high-performance liquid chromatography/quadrupole- time-of-flight mass spectrometry combined with a screening procedure. Rapid Commun. Mass Spectrom. 2014, 28, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Liu, X.-B.; Wu, H.-M.; Cheng, P.; Liu, Y.-S.; Zeng, J.-G. An improved separation method for classification of Macleaya cordata from different geographical origins. Anal. Methods 2015, 7, 1866–1871. [Google Scholar] [CrossRef]
- Xie, H.; Yang, J.; Feng, S.; Cheng, P.; Zeng, J.; Xiong, X. Simultaneous quantitative determination of sanguinarine, chelerythrine, dihydrosanguinarine and dihydrochelerythrine in chicken by HPLC-MS/MS method and its applications to drug residue and pharmacokinetic study. J. Chromatogr. B 2015, 985, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Cheng, P.; Liu, X.-B.; Liu, Y.-S.; Zeng, J.-G. Systematic identification of alkaloids in Macleaya microcarpa fruits by liquid chromatography tandem mass spectrometry combined with the isoquinoline alkaloids biosynthetic pathway. J. Pharm. Biomed. Anal. 2015, 103, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Qing, Z.; Liu, S.; Liu, W.; Xie, H.; Zeng, J. Regiospecific Minisci acylation of phenanthridine via thermolysis or photolysis. Tetrahedron Lett. 2014, 55, 6647–6651. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Xie, H.; Liu, W.; Zeng, J.; Cheng, P. A novel C–C radical–radical coupling reaction promoted by visible light: Facile synthesis of 6-substituted N-methyl 5,6-dihydrobenzophenanthridine alkaloids. RSC Adv. 2016, 6, 50500–50505. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4 and 7 are available from the authors.


| Entry | Photocatalyst | Terminal Reductant | Solvent | Yield of 4a b |
|---|---|---|---|---|
| 1 | Ru(bpy)3Cl2·6H2O | - | MeCN | 6% |
| 2 | Ru(bpy)3Cl2·6H2O | i-Pr2NEt | MeCN | 32% |
| 3 | Ru(bpy)3Cl2·6H2O | i-Pr2NEt | DMSO | 37% |
| 4 | Ru(bpy)3Cl2·6H2O | i-Pr2NEt | DMF | 46% |
| 5 | Ir(ppy)3 | - | DMF | 28% |
| 6 | Ir(ppy)3 | i-Pr2NE | DMF | 51% |
| 7 | eosin Y | - | DMF | 75% |
| 8 | eosin Y | i-Pr2NEt | DMF | 74% c |
| 9 | eosin Y | i-Pr2NEt | DMF | 0% d |
| 10 | - | i-Pr2NEt | DMF | 7% |
| 11 | - | i-Pr2NEt | DMF | 0% d |
| 12 | - | - | DMF | trace e |
| Substrate | Product | Substrate | Product |
|---|---|---|---|
 |  |  |  |
| 3a | 4a 74% | 3b | 4b 51% |
 |  |  |  |
| 3c | 4c 46% | 3d | 4d 49% |
 |  |  |  |
| 3e | 4e 53% | 3f | 4f 80% |
 |  + +  |  |  + +  |
| 3g | 4ga 33% 4gb 17% | 3h | 4ha 38% 4hb 20% |
 |  |  |  |
| 3i | 4i 51% | 3j | 4j 46% |
 |  |  |  |
| 3k | 4k 40% | 3l | 4l 49% |
 |  |  |  |
| 3m | 4m 53% | 3n | 4n 46% |
 |  |  |  |
| 3o | 4o 43% | 3p | 4p 57% |
 |  |  |  |
| 3q | 4q 49% | 3r | 4r 43% |
| Substrate | Product | Substrate | Product |
|---|---|---|---|
 |  |  |  |
| 6a | 7a 92% | 6b | 7b 89% |
 |  |  |  |
| 6c | 7c 88% | 7d | 7d 91% |
 |  |  |  |
| 6e | 7e 85% | 6f | 7f 90% |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Qing, Z.; Cheng, P.; Zheng, X.; Zeng, J.; Xie, H. Metal-Free Photoredox Catalyzed Cyclization of O-(2,4-Dinitrophenyl)oximes to Phenanthridines. Molecules 2016, 21, 1690. https://doi.org/10.3390/molecules21121690
Liu X, Qing Z, Cheng P, Zheng X, Zeng J, Xie H. Metal-Free Photoredox Catalyzed Cyclization of O-(2,4-Dinitrophenyl)oximes to Phenanthridines. Molecules. 2016; 21(12):1690. https://doi.org/10.3390/molecules21121690
Chicago/Turabian StyleLiu, Xiubin, Zhixing Qing, Pi Cheng, Xinyu Zheng, Jianguo Zeng, and Hongqi Xie. 2016. "Metal-Free Photoredox Catalyzed Cyclization of O-(2,4-Dinitrophenyl)oximes to Phenanthridines" Molecules 21, no. 12: 1690. https://doi.org/10.3390/molecules21121690