Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Fermentation Time on Pigment Production MP-Fermented Broth (MFB)
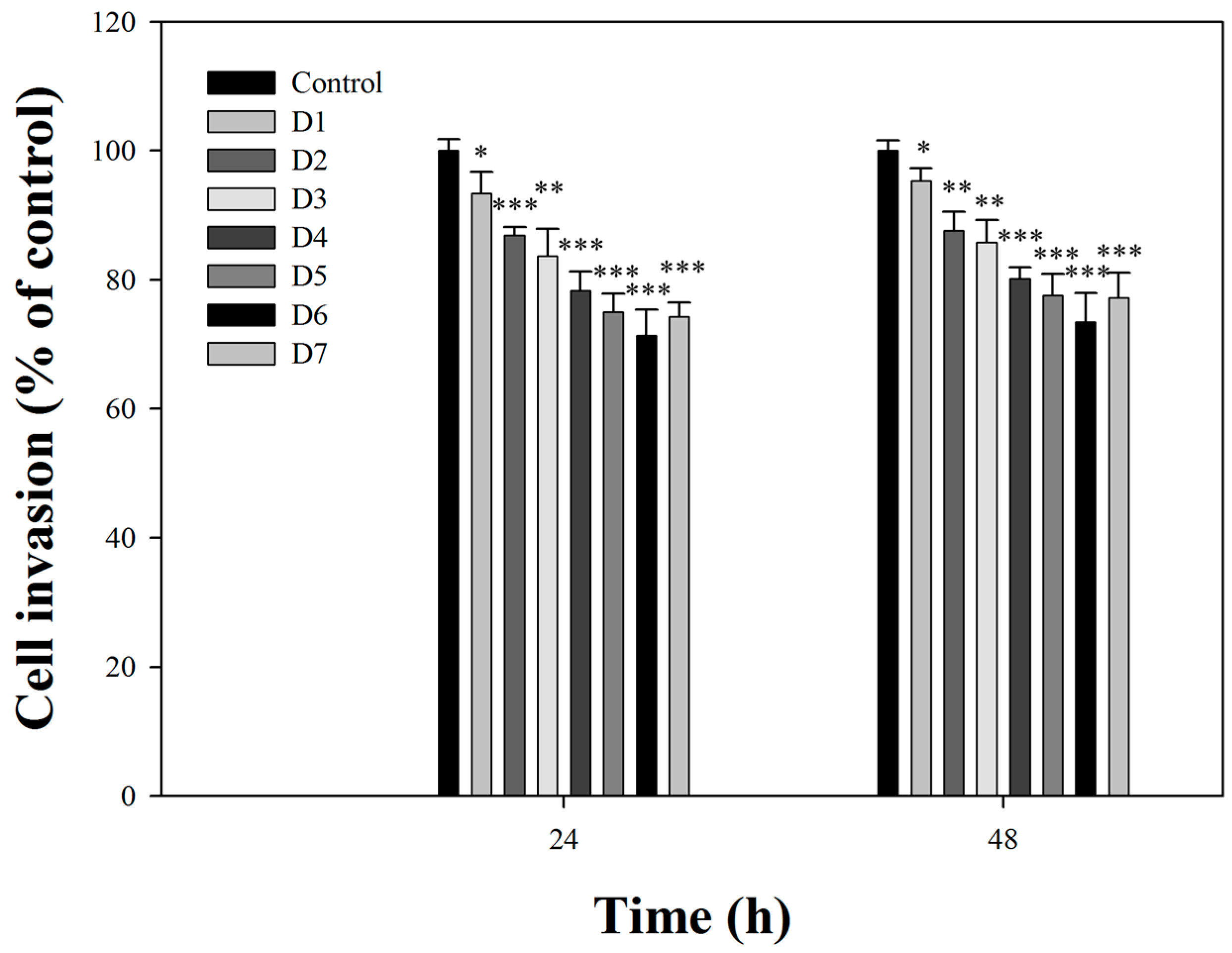
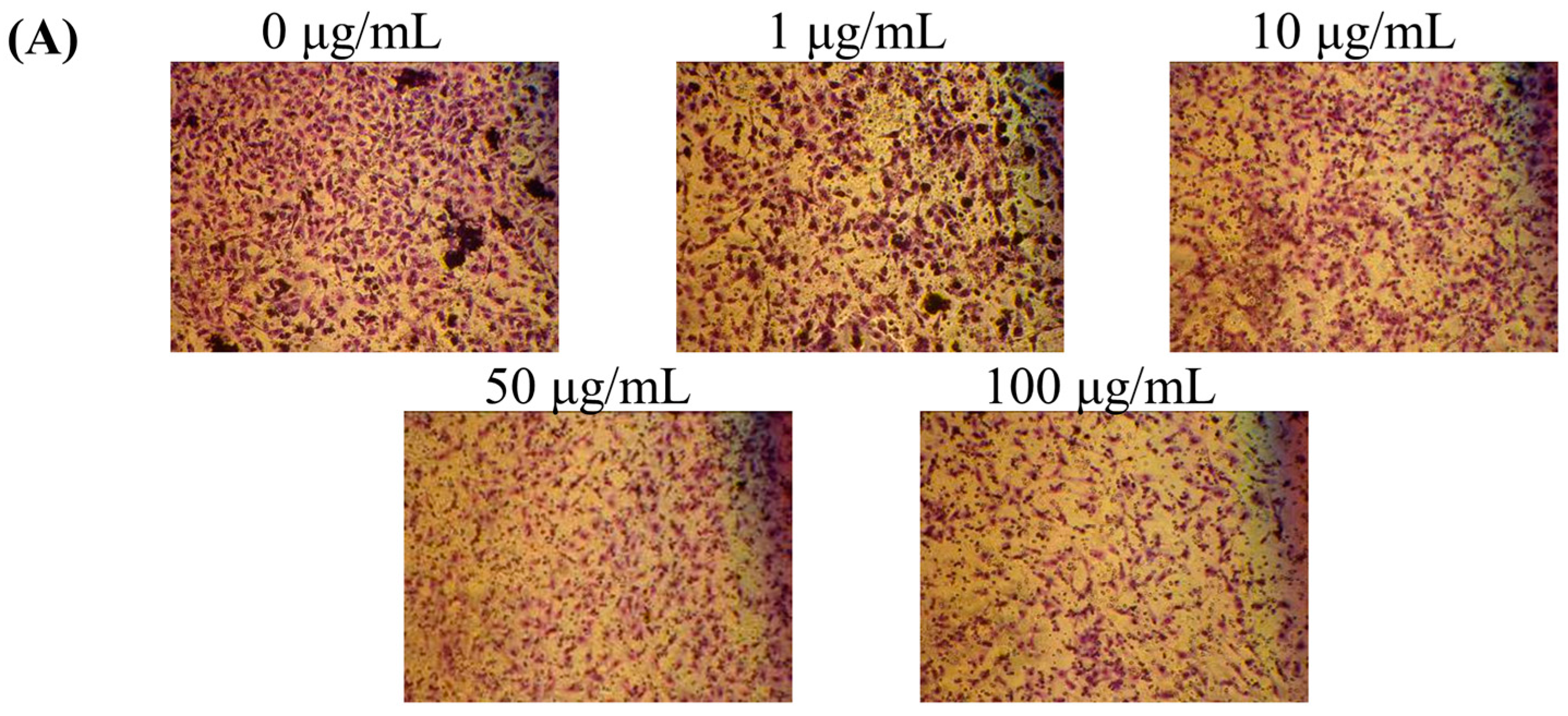
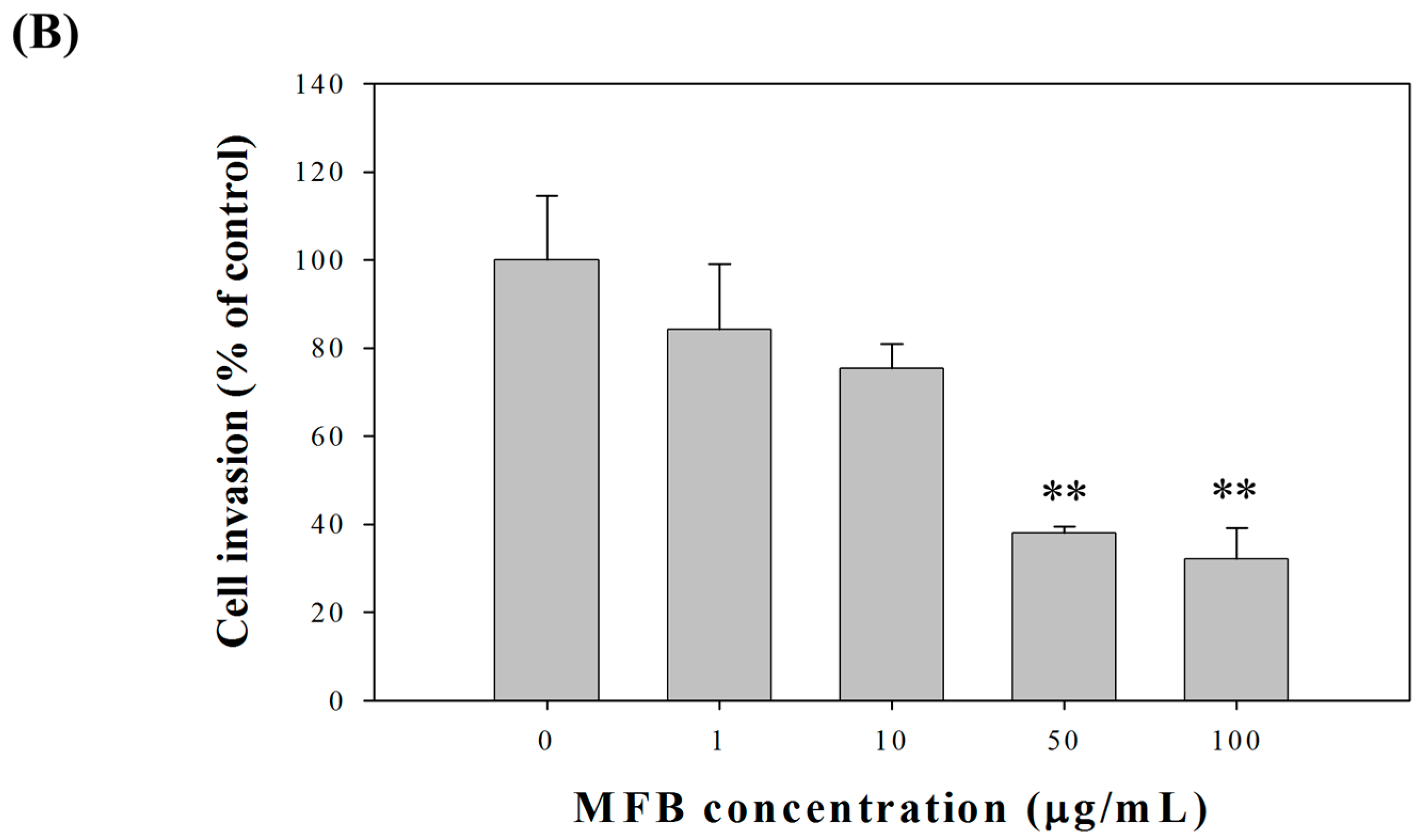
2.2. Effects of Fermentation Time of MFB on In Vitro Cell Invasion
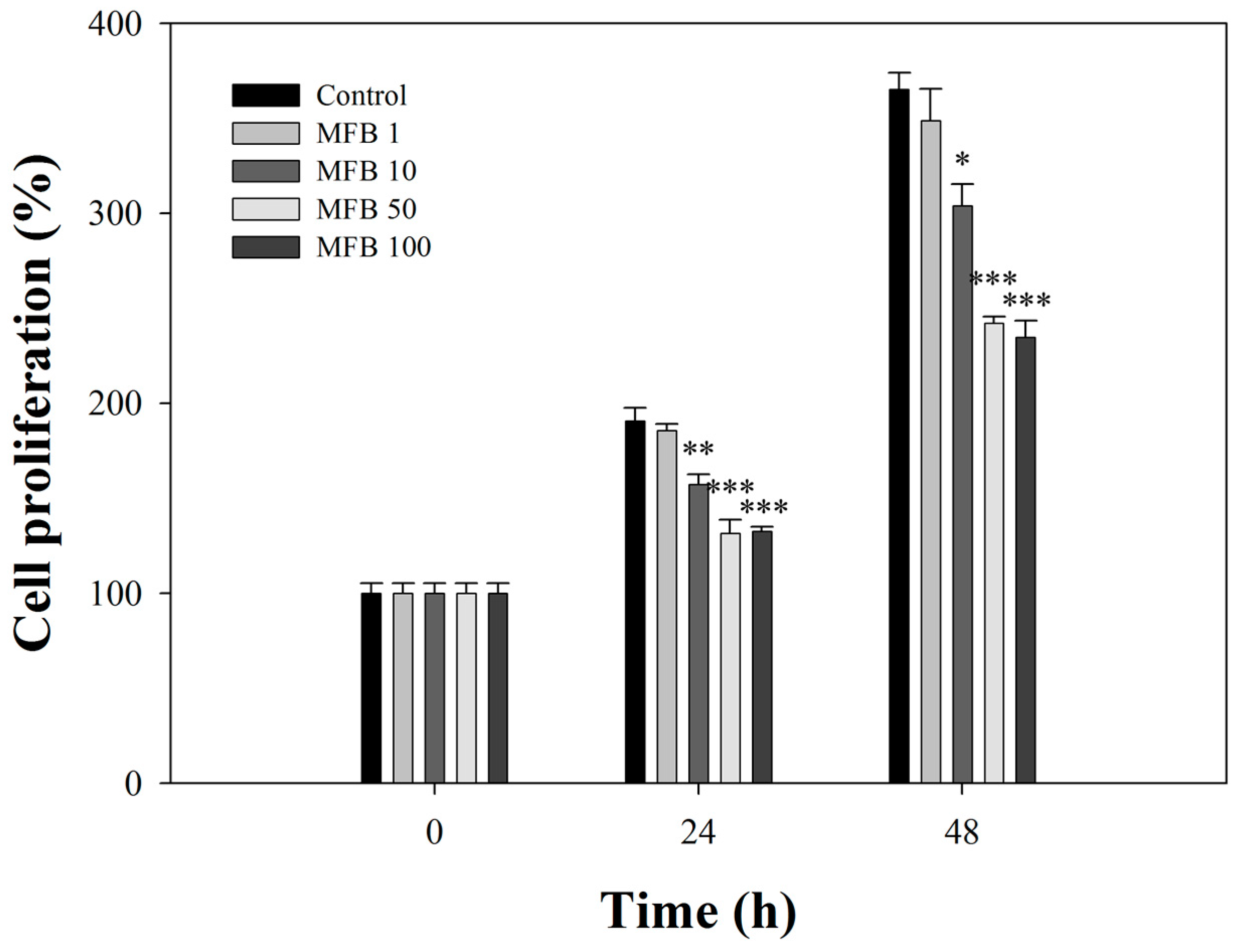
2.3. Effects of MFB on the Proliferation of SK-Hep-1 Cells
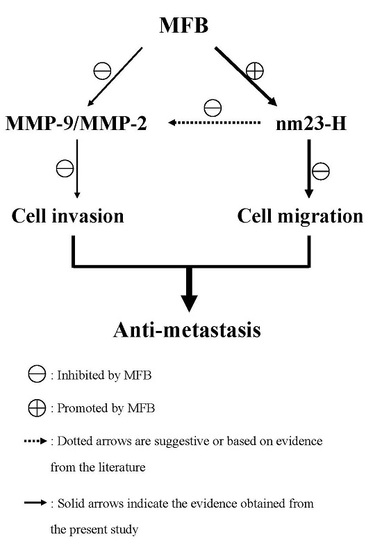
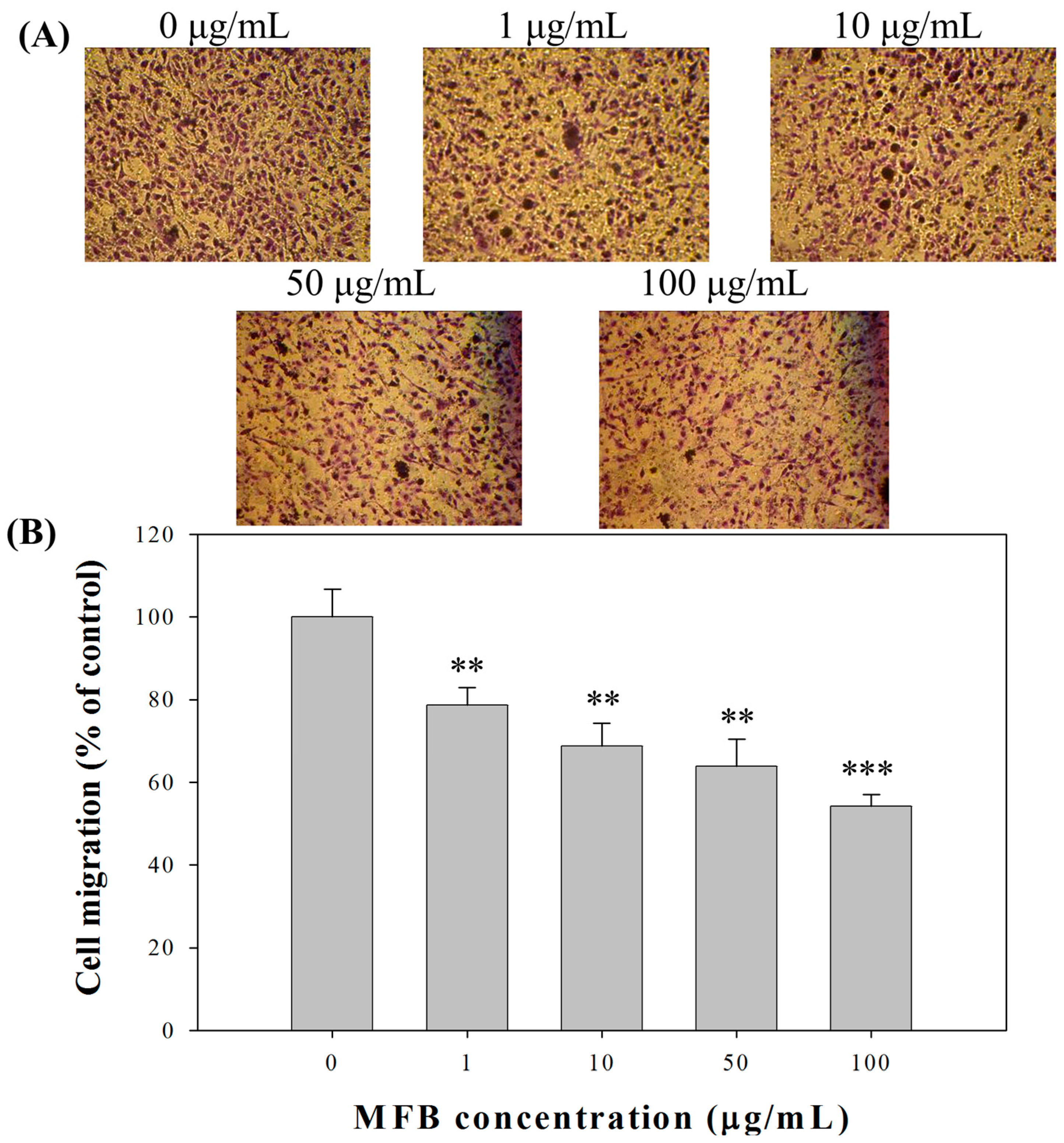
2.4. Effect of MFB on In Vitro Invasion and Migration of SK-Hep-1 Cells
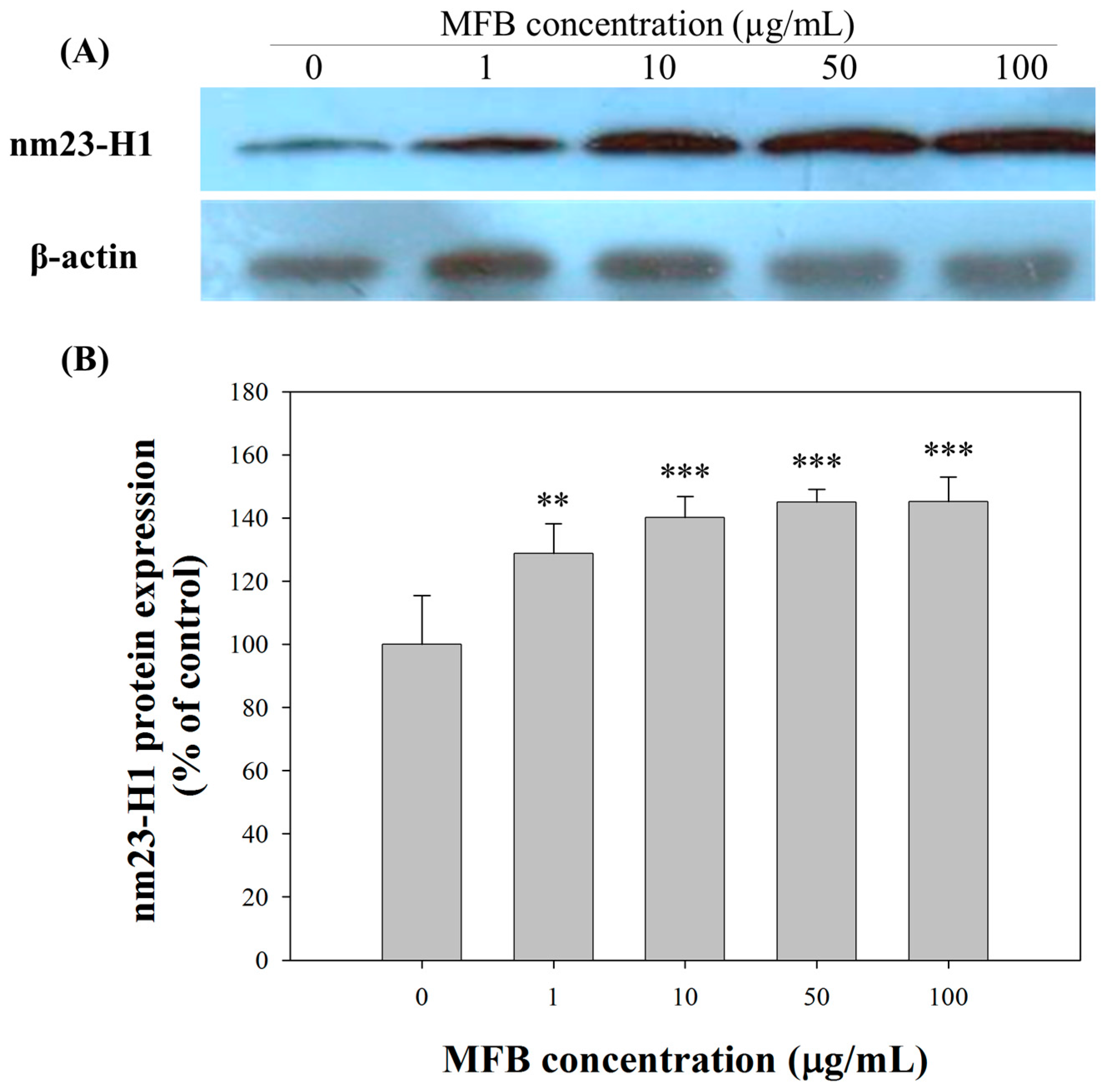
2.5. Upregulation of nm23-H1 by MFB at the Protein Level
2.6. Effects of MFB on the Activities of MMP-9 and MMP-2
3. Materials and Methods
3.1. Cell Line and Chemicals
3.2. Isolation and Screening of an Antioxidant-Producing Strain
3.3. Extract Preparation
3.4. Cell Culture and Cell Proliferation
3.5. Cell Migration Assay
3.6. Cell Invasion Assay
3.7. Gelatin Zymography
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco‘s Minimal Essential Medium |
| DSR | Distiller’s sorghum residue |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| LLC | Lewis lung carcinoma |
| MMP | Matrix metalloproteinase |
| MFB | Monascus purpureus-fermented broth |
| MP | Monascus purpureus CWT715 |
| SDS | Sodium dodecyl sulfate |
References
- Fidler, I.J.; Gersten, D.M.; Hart, I.R. The biology of cancer invasion and metastasis. Adv. Cancer Res. 1978, 28, 149–250. [Google Scholar] [PubMed]
- Steeg, P.S.; Bevilacqua, G.; Kopper, L.; Thorgeirsson, U.P.; Talmadge, J.E.; Liotta, L.A.; Sobel, M.E. Evidence for a novel gene associated with low tumor metastatic potential. J. Natl. Cancer Inst. 1988, 80, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Hartsough, M.T.; Clare, S.E.; Mair, M.; Elkahloun, A.G.; Sgroi, D.; Osborne, C.K.; Clark, G.; Steeg, P.S. Elevation of breast carcinoma nm23-H1 metastasis suppressor gene expression and reduced motility by DNA methylation inhibition. Cancer Res. 2001, 61, 2320–2327. [Google Scholar] [PubMed]
- Khan, M.H.; Yasuda, M.; Higashino, F.; Haque, S.; Kohgo, T.; Nakamura, M.; Shindoh, M. NM23-H1 suppresses invasion of oral squamous cell carcinoma-derived cell lines without modifying matrix metalloproteinase-2 and matrix metalloproteinase-9 expression. Am. J. Pathol. 2001, 158, 1785–1791. [Google Scholar] [CrossRef]
- Huang, C.S.; Shih, M.K.; Chuang, C.H.; Hu, M.L. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J. Nutr. 2005, 135, 2119–2123. [Google Scholar] [PubMed]
- Boissan, M.; de Wever, O.; Lizarraga, F.; Wendum, D.; Poincloux, R.; Chignard, N.; Desbois-Mouthon, C.; Dufour, S.; Nawrocki-Raby, B.; Birembaut, P.; et al. Implication of metastasis suppressor nm23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res. 2010, 70, 7710–7722. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Xu, B.; He, M.; Lan, X.; Wang, Q. Nm23-H1 suppresses hepatocarcinoma cell adhesion and migration on fibronectin by modulating glycosylation of integrin beta1. J. Exp. Clin. Cancer Res. 2010, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Liabakk, N.B.; Talbot, I.; Smith, R.A.; Wilkinson, K.; Balkwill, F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenasesin colorectal cancer. Cancer Res. 1996, 56, 190–196. [Google Scholar] [PubMed]
- Arii, S.; Mise, M.; Harada, T.; Furutani, M.; Ishigami, S.; Niwano, M.; Mizumoto, M.; Fukumoto, M.; Imamura, M. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology 1996, 24, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ra Yoon, M.; Hyun Nam, S.; Young Kang, M. Antioxidative and antimutagenic activities of 70% ethanolic extracts from four fungal mycelia-fermented specialty rices. J. Clin. Biochem. Nutr. 2008, 43, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.Y.; Pan, T.M. The Monascus metabolite monacolin K reduces tumor progression and metastasis of Lewis lung carcinoma cells. J. Agric. Food Chem. 2009, 57, 8258–8265. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.Y.; Wu, Y.M.; Chang, K.J.; Pan, T.M. Dimerumic acid inhibits SW620 cell invasion by attenuating H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int. J. Biol. Sci. 2011, 7, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.C.; Pan, T.M. Beneficial effects of Monascus purpureus NTU 568-fermented products: A review. Appl. Microbiol. Biotechnol. 2011, 90, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Lin, Y.L.; Lee, M.H.; Ho, C.Y. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 2005, 53, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xin, Y.; Shi, X.; Guo, Y. Anti-cancer effect of rubropunctatin against human gastric acrcinoma cells BGC-823. Appl. Microbiol. Biotechnol. 2010, 88, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Seeram, N.P.; Zhang, Y.; Heber, D. Chinese red yeast rice versus lovastatin effects on prostate cancer cells with and without androgen receptor overexpression. J. Med. Food 2008, 11, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Hu, H.H.; Tsai, Y.M.; Chang, W.T. In vitro effects of Monascus purpureus on antioxidation activity during fermentation of Kinmen sorghum liquor waste. J. Biosci. Bioeng. 2013, 115, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xin, Y.; Shi, X.; Guo, Y. Cytotoxicity of Monascus pigments and their derivatives to human cancer cells. J. Agric. Food Chem. 2010, 58, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Hung, C.M.; Chen, Y.L.; Wu, M.D.; Yuan, G.F.; Wang, Y.J. Monascuspiloin induces apoptosis and autophagic cell death in human prostate cancer cells via the Akt and AMPK signaling pathways. J. Agric. Food Chem. 2012, 60, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.Y.; Wu, Y.M.; Hsu, Y.W.; Hsu, L.C.; Kuo, Y.H.; Chang, K.J.; Pan, T.M. Effects of Monascus-fermented rice extract on malignant cell-associated neovascularization and intravasation determined using the chicken embryo chorioallantoic membrane model. Integr. Cancer Ther. 2010, 9, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yung, L.Y.; Wong, Y.H. Metastasis suppressors nm23H1 and nm23H2 differentially regulate neoplastic transformation and tumorigenesis. Cancer Lett. 2015, 361, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Meneses, P.I.; Lan, K.; Robertson, E.S. The suppressor of metastasis nm23-H1 interacts with the Cdc42 Rho family member and the pleckstrin homology domain of oncoprotein Dbl-1 to suppress cell migration. Cancer Biol. Ther. 2008, 7, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Iwashita, S.; Yamaguchi, S.; Ono, Y. Role of nm23 in the regulation of cell shape and migration via Rho family GTPase signals. Mol. Cell. Biochem. 2009, 329, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Keely, P.J.; Westwick, J.K.; Whitehead, I.P.; Der, C.J.; Parise, L.V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 1997, 390, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell. Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Fan, Y.E.; Lin, C.Y.; Hu, M.L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J. Nutr. Biochem. 2007, 18, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sze, K.M.; Wong, K.L.; Chu, G.K.; Lee, J.M.; Yau, T.O.; Ng, I.O. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology 2011, 53, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Pan, X.; Zhang, Z.; Gao, J.; Zhang, L.; Chen, J. Downregulation of integrin-linked kinase inhibits epithelial-to-mesenchymal transition and metastasis in bladder cancer cells. Cell. Signal. 2012, 24, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Miyata, Y.; Koga, S.; Kanda, S.; Kanetake, H. Expression of nm23-H1 gene product in sarcomatous cancer cells of renal cell carcinoma: Correlation with tumor stage and expression of matrix metalloproteinase-2, matrix metalloproteinase-9, sialyl Lewis X, and c-erbB-2. Urology 2005, 65, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Yang, S.F.; Chen, G.D.; Han, C.P.; Chen, S.C.; Lin, L.Y.; Ko, J.L. Human nonmetastatic clone 23 type 1 gene suppresses migration of cervical cancer cells and enhances the migration inhibition of fungal immunomodulatory protein from Ganoderma tsugae. Reprod Sci. 2007, 14, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Repesh, L.A. A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis 1989, 9, 192–208. [Google Scholar] [PubMed]
- Sample Availability: Not available.

| Sample | OD400nm (yellow) | OD470nm (orange) | OD500nm (red) |
|---|---|---|---|
| Day 1 | 1.0 | 1.0 | 1.0 |
| Day 2 | 1.4 | 1.4 | 1.4 |
| Day 3 | 2.3 | 2.3 | 2.5 |
| Day 4 | 4.4 | 4.7 | 5.3 |
| Day 5 | 4.5 | 5.2 | 6.0 |
| Day 6 | 4.7 | 5.2 | 5.8 |
| Day 7 | 4.0 | 4.6 | 5.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-T.; Chuang, C.-H.; Lee, W.-J.; Huang, C.-S. Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells. Molecules 2016, 21, 1691. https://doi.org/10.3390/molecules21121691
Chang W-T, Chuang C-H, Lee W-J, Huang C-S. Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells. Molecules. 2016; 21(12):1691. https://doi.org/10.3390/molecules21121691
Chicago/Turabian StyleChang, Wen-Teish, Cheng-Hung Chuang, Wan-Ju Lee, and Chin-Shiu Huang. 2016. "Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells" Molecules 21, no. 12: 1691. https://doi.org/10.3390/molecules21121691