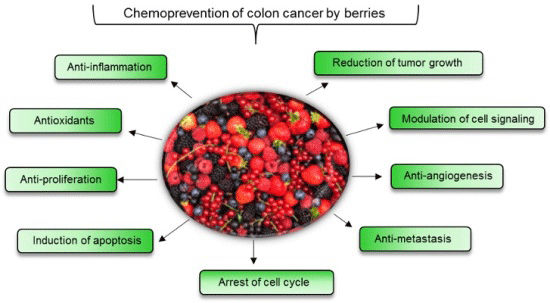
Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment
Abstract
:1. Introduction

2. Bioactive Compound Profile and Antioxidant Capacity of Berries
| Berries | Major Bioactive Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Flavonoids | Phenolic Acids | Tannins | Vitamins | Stilbenes | Other Compounds | References | |
 | Anthocyanins (Cyanidin glycosides, cyaniding-3-arabinose, cyanidin-3-soporoside, Cyanidin-3-rutinoside, and pelargonidin glycosides), quercitin, catechin, epicatechin, apigenin, chrysin and naringenin | Caffeic acid, ferulic acid, gallic acid, chlorogenic acid, p-coumaric acid and p-hydroxybenzoic acid | Ellagitannin and ellagic acid | Folate, Vitamin C and B | Resveratrol | Polyunsaturated fatty acids, calcium, potassium, magnesium, phosphorus, lutein, α and β carotene | [60,61] |
 | Anthocyanins (malvidin glycosides, cyanidin glycosides, delphinidin glycosides and petunidin glycosides), myricetin glycosides, quercetin glycosides, kaempferol, (+)-catechin and (−)-epicatechin | Benzoic and cinnamic acids | Proanthocyanidins | Vitamin C, B complex, E, A and ascorbic acid | Pterostilbene | Potassium, calcium, magnesium, phosphorus, β-carotene and lutein | [62,63] |
 | Anthocyanins (malvidin-3-glucoside, peonidin-3-glucoside, cyanidin-3-glucoside and petunidin-3-glucoside), quercetin, kaempferol, (+)-catechin, epicatechin and epicatechin gallate | Hydroxycinnamic acid, gallic acid, caffeic acid, coumaric acid and ferulic acid | Proanthocyanidins and ellagic acid | Vitamin C and K | Resveratrol, pterostilbene, piceid, viniferins, astringin and piceatannol | Copper, carotenoids (β-carotene and lutein), and melatonin | [64,65] |
 | Anthocyanins (cyanidin-3-glucoside, pelargonidin and pelargonidin-3-rutinoside), quercetin glycosides, kaempferol glycosides and flavan-3-ols ((+)-catechin) | Hydroxycinnamic acids, gallic acid, caffeic acid, p-coumaric acid and coumaroyl glycosides | Proanthocyanidins, ellagitannins, gallotannins, ellagic acid and its glycosides. | Folate and Vitamin C | Resveratrol | Potassium, calcium, magnesium and phosphorus | [66,67] |
 | Anthocyanins(cyanidin glycosides, peonidin glycosides, pelargonidin glycosides, malvidin glycosides, delphinidin glycosides) kaempferol and quercetin | p-Coumaric acid and hydroxycinnamic acid | Proanthocyanidins | Folate, Vitamin C and A | Resveratrol | Calcium, iron, potassium, magnesium and mamganese | [61,68] |
 | Anthocyanin (cyanidin-3-galactoside, cyanidin-3-glucoside and cyanidin-3-arabinoside) , quercetin | Chlorogenic acid, Caffeic acid derivative | Proanthocyanidins | Ascorbic acid | Resveratrol | Carotenoids, sterols and lipids | [69,70] |
 | p-Hydroxybenzoic acid, m-hydroxybenzoic and 3,4-dihydroxy-mandelic | Proanthocyanidins | Folate | α-Mangostin, β-Mangostin, µ-Mangostin, 1,3,6,7-Tetrahydroxy Xanthone, 1-Isomangostin, Mangosharin, calcium, potassiuum and magnesium | [71,72,73,74] | ||
 | Anthocyanins (cyanidin glycosides, pelargonidin glycosides, peonidin glycosides), quercitin, cyaniding and epicatechin | Gentisic acid, protocatchiuic acid, salicylic acid and caffeic acid | Ellagitannins and ellagic acid | Folate and Vitamin C (ascorbic acid) | β-carotene, cryptoxanthin and lutein | [60] | |
 | Anthocyans (delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside), catechins, quercetin, myricetin and kaempferol | Gallic acid, p-hydroxy-benzoic acid and hydroxycinnamic acid | Proanthocyanidin, ellagitannins and gallotannins | Vitamin A and B2 | Stilbenoids | Calcium, zinc, magnesium, potassium, gibberellic acids and γ-linolenic acid | [75] |
 | Anthocyans (cyanidin-3-galactoside and cyanidin-3-arabinoside, cyanidin-3-galactoside and cyanidin-3-arabinoside), quercetin glycosides and flavan-3-ols ((−)-epicatechin) | Caffeic acid, hydroxycinnamic acids, chlorogenic acid and neochlorogenic acids | Proanthocyanidins | Vitamin B and C | Stilbenes | Potassium and zinc, β-carotene and β-cryptoxanthin | [33,76,77] |
 | Anthocyanins, flavonols and flavan-3-ols | p-Coumaric acid, hydroxycinnamic acids, caffeic acid, ferulic acid and gallic acid | Poanthocyanidins, ellagitannins and ellagic acid | Vitamin C and α-tocopherol | Stilbenes | β-carotene | [78,79,80] |
 | Isorhamnetin (isorhamnetin-rutinoside, isorhamnetin-glycosid) quercetin-rutinoside, quercetin-glycoside and kaempferol | Hydroxyursolic acid | Vitamin A, B2, C and E | Carotenoid, calcium, magnesium, potassium and sodium | [81,82] | ||
 | Anthocyanins (cyanidin-3-O-sambubioside, delphinidin-3-O-galactoside, peonidin-3-O-galactoside), quercetin-3-galactoside and flavan-3-ols ((−)-epicatechin) | Ferulic acid, benzoic acid and phenylacetic acid | Proanthocyanidins | Vitamin C and E | Trans-resveratrol | [83,84,85] | |
 | Chrysanthemin, hyperoside, pelargonin and petunidin-3-O-beta-d-glucoside | Chlorogenic acid | Tannin | Ascorbic acid and Vitamin K | Isoquinoline alkaloids (berberine, berbamine and palmatine), and β-carotene | [86] | |
 | Anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, and peonidin), isovitexin ,luteolin, quercetin, dihydrokaempferol, chrysoerial and flavan-3-ols | Protocatechuic acid, ferulic acid, syringic acid and vanillic acid | Resveratrol | [87] | |||
 | Myricetin, quercetin and kaempferol | p-Coumaric acid | Vitamin B1, B2, B3, B6, C and E | L. barbarum polysaccharides, amino acids, zinc, iron, copper, calcium, germanium, selenium, phosphorus and β-carotene | [88] | ||
 | Myricetin and epigallocatechin gallate | Phenolcarboxylic acids, benzoic acid, cinnamic acid and caffeic acid | Condensed tannins | Ascorbic acid | Lycopene, linoleic acid, oleic acid, and stearic acid | [89] | |
 | (+)-catechin, and quercetin aglycon | Hydroxybenzoic acid derivative and hydroxycinnamic acid derivatives | Proanthocyanidins | [90] | |||
 | Anthocyanins (cyanidin 3-glucoside, cyanidin 3-rutinoside), (+)-catechin, (−)-epicatechin, quercetin 3-glucuronide and isorhamnetin 3-glucuronide. | Hydroxycinnamic acids | Ellagitannins, ellagic acid and its derivatives | [78] | |||
 | Anthocyanins (cyanidin 3,5-diglucoside and cyanidin 3-glucoside) | Chlorogenic acid | Vitamin C | Lectins | [91] | ||
 | Anthocyanins (3,5-diglucosides of delphinidin, petunidin and malvidin), dihydroquercetin diglucoside, myricetin | Gallic acid and galloyl-glucose ester | Carotenoids and lutein | [92] | |||
 | Quercetin, kaempferol and myricetin | Gallic acid, protocatechuic acid, syringic acid, coumaric acid and vanillic acid | Ellagic acid | Vitamin C | Ascorbate, β-carotene, glutathione and α-tocopherol | [93] | |
 | Quercetin | Gallic acid, chebulagic acid, 3-ethyl-gallic acid and geraniin | Ellagic acid, corilagin, and isocorilagin | Vitamin C | Galloyl glucose, amino acids and minerals | [94,95] | |
| Berries | Antioxidant Capacity (µmol Trolox Equivalents/g) | References |
|---|---|---|
| Chokeberry | 158.2 | [101,102] |
| Raspberry | 21.4 | [103] |
| Lowbush blueberry | 64.4 | [103] |
| Elderberry | 145.0 | [102] |
| Blackberry | 55.7 | [104] |
| Rabbiteye blueberry | 123.4 | [104] |
| Black currant | 56.7 | [104] |
| Lingonberry | 38.1 | [101] |
| Cranberry | 18.5 | [101] |
| Red grape | 7.4 | [105] |
| White grape | 4.5 | [105] |
| Strawberry | 53.03 | [106] |
| Jamunberry | 16.4 | [92] |
| Emblic | 134.33 | [95] |
3. Bioavailability and Metabolites of Berries

4. Biological Activities of Berries against Colon Cancer: in Vitro and in Vivo Animal Studies
4.1. Raspberry
| Berry Extracts/Fraction/Component | Model (Cell Lines or Animal) | Duration and Dose/Intervention | Effects on Colon Cancer | References |
|---|---|---|---|---|
| Raspberry | ||||
| In vitro | ||||
| Polyphenolic-rich extracts | HT-29 and HCT-115 cells | 0, 3.125, 6.25, 12.5, 25, 50 μg/mL for 24 h | -Inhibit initiation, promotion and invasion. | [131] |
| Anthocyanins rich extracts of black raspberry | HCT-116, Caco-2 and SW480 cells | 0.5, 5, and 25 μg/mL for 3 days | -Inhibit proliferation. -Suppress DNMT1 and DNMT3B proteins. -Suppress downstream of Wnt pathway. -Induce apoptosis. | [130] |
| ET and their derivatives from black raspberry seeds | HT-29 cells | 5 to 30 μg/mL for 24 and 48 h | -Arrest cell cycle. -Induce apoptosis by extrinsic and intrinsic pathways. | [132] |
| Aqueous extracts of black raspberry | HT-29 cells | 0 to 400 µg/mL for 24 to 48 h | -Inhibit cancer cell growth -Induce apoptosis. | [133] |
| Red raspberry extracts | LoVo cells | 5%, 7.5%, and 10% for 24 to 48 h | -Reduce the survival of cells. | [134] |
| Black raspberry extracts | HT-29 and HCT-116 cells | 25–200 µg/mL for 48 h | -Induce cytotoxic effects. | [59] |
| Gastrointestinal digestion and colonic fermentation | HT-29 and HT-115 cells | 0–50 µg/mL gallic acid equivalents (GAE) for 24 h. | -Exert anti-genotoxic, anti-mutagenic and anti-invasive activity. | [116] |
| Freeze-dried extracts from black raspberry | HT-29 cells | 0.6 and 1.2 mg of extract/mL for 48 h | -Retain their anticancer activity after digestion. | [135] |
| In vivo | ||||
| Lyophilized black raspberry | AOM induced Fischer 344 rat | 0%, 2.5%, 5%, or 10% (wt/wt) for 9 to 33 weeks | -Decrease the multiplicity of ACF, total tumors, adenomas, and adenocarcinomas. | [136] |
| Black raspberry extracts | Interleukin-10 knock-out mouse | 5% for 8 weeks | -Decrease colonic ulceration. | [137] |
| Freeze-dried black raspberry | Apc1638+/− mice and Muc2−/− mice | 10% for 12 weeks | -Lower tumor incidence and multiplicity. | [138] |
| DSS induced male C57BL/6J mice | 5%–10% for 7–14 days | -Ameliorates ulcerative colitis. -Suppresses inflammation. | [139] | |
| DSS induced male C57BL/6J mice | 5% for 28 days | -Suppresses colonic ulceration by correcting promoter hypermethylation of suppressor genes. | [140] | |
| Blueberry | ||||
| In vitro | ||||
| Dried extracts and fractions | HT-29 and Caco-2 cells | 50–10,000 µg/mL for 48 h | -Inhibit cancer cell proliferation. -Induce apoptosis. | [141] |
| Ethanol/water extracts | HT-29 cells | 0.025%–0.5% dry wt for 24 h | -Exert antiproliferative activity. | [142] |
| Anthocyanin-rich extracts | Caco-2 cells | 0.1–100 nM for 1 h | -Act as an intracellular antioxidant. | [143] |
| DLD-1 and COLO205 cells | 50–250 μg/mL for 24 h | -Repress the proliferation. -Induce apoptosis. | [144] | |
| Blueberry extracts | HT-29 and HCT116 cells | 25–200 µg/mL for 24 to 48 h | -Inhibit cancer cell proliferation. | [145] |
| IVD and colonic fermentation | HT-29 or CRL-1790 cells | 10, 25, 50, 75 or 100 µg/mL for 24 to 48 h | -Alter antiproliferative and antioxidant activity after digestion. | [146] |
| Delphinidin | HCT116 cells | 30–240 mM for 48 h | -Inhibit cancer cell growth. -Induce apoptosis. -Arrest cell cycle. | [147] |
| Anthocyanin-enriched fractions | HT-29 cells | 50–150 µg/mL for 6 h | -Induce apoptosis. | [148] |
| Pterostilbene | HT-29 cells | 50 µM for 4 h | -Suppresses cell growth. -Suppresses inflammation. | [149] |
| In vivo | ||||
| Pterostilbene | AOM induce Fisher 344 male | 40 p.p.m. (0.004%) for 45 weeks | -Reduce tumor multiplicity, by inhibiting the Wnt/β-catenin signaling pathway. | [149] |
| Blueberry extracts | AOM induce Fisher 344 male | 50 g/kg for 13 weeks | -Reduce formation of AOM-induced ACF and increase in hepatic GST activity. | [150] |
| Blueberry husks and mixture of three probiotic | DSS treatment rat | 50 g /kg diet for 6 months | -Reduce colonic ulcers and dysplastic lesions. | [151] |
| Grape | ||||
| In vitro | ||||
| Grape seed proanthocyanidin extract | Caco-2 cells | 10–100 µg/mL for 24 h | -Inhibits cancer cell proliferation. -Reduces PI3k/PKB signaling pathway. -Induces caspase-3 dependent apoptosis. | [152] |
| Anthocyanin-rich extracts | HT-29 cells | 0 to 200 μg/mL for 48 h | -Inhibit cell proliferation. | [153] |
| HT-29 cells | 10–75 µg of monomeric anthocyanin/mL for 24–72 h | -Induce anti-proliferative activity. | [154] | |
| HT-29 cells | 500 µg/ml for 72 h | -Protect DNA damaging properties of topoisomerase poisons. | [155] | |
| Obacunone and obacunone glucoside (OG) from seeds of marsh white grape | SW480 cells | 6.25, 12.5, 50, and 100 µM for 24, 48 and 72 h | -Induce intrinsic pathway of apoptosis. | [156] |
| Grape waste | Caco-2 cells | 0.5, 1.5, 10, 50, or 100 mL/L for 24 h | -Induce strong antiradical and antiproliferative activity. -Arrest cells cycle. | [157] |
| In vivo | ||||
| Anthocyanin-rich extracts | AOM treated Fischer 344 male rats | 3.85 g of monomeric anthocyanin/kg body weight for 14 weeks | -Inhibit colonic aberrant crypt foci formation. | [158] |
| Total pholyphenolic extracts | DMH induced F344 rats | 0.11 % (w/w) for 16 weeks | -Decrease number of adenomas. | [159] |
| Proanthocyanidin-rich dietary fiber | C57BL/6J mice | 10 mL/kg body weight for 2 weeks | -Alters the expression of tumor suppressor genes and proto-oncogenes. -Modulates genes associated with lipid biosynthesis, energy metabolism, cell cycle, and apoptosis. | [160] |
| Strawberry | ||||
| In vitro | ||||
| Crude extracts and purified compounds | HT-29 and HCT-116 cells | 250 μg/mL (crude extract) and 100 μg/mL (pure compounds) for 48 h | -Inhibit cell proliferation. | [161] |
| Polyphenol-rich extracts | Caco-2 cells | 25, 50, and 75 μg of GAE /mL | -Show anti neoplastic activity. | [162] |
| Strawberry extracts | HT-29 cells | 0.025%, 0.05%, 0.25%, 0.5% for 24 h | -Organically grow strawberry extracts show higher antiproliferative activity. | [163] |
| HT-29 and HCT-116 cells | 25–200 µg/mL for 24 to 72 h | -Inhibit cancer cell proliferation. | [145] | |
| IVD and fecal fermentation | HT-29 and HT-115 cells | 0–50 µg/mL GAE for 24 h | -Exerts anti-genotoxic, anti-mutagenic and anti-invasive activity. | [116] |
| Extracts from strawberries treated with essential oils | HT-29 cells | 3 mg/mL for 24 h to 96 h | -Exhibit strong radical scavenging capacity and antiproliferative activity. | [164] |
| Kaempferol | HT-29 cells | 0 or 60 μmol/L for 24 to 72 h | -Inhibit cancer cells growth. -Arrest cell cycle. | [165] |
| ET extracts, EA and UA. | Human 293T cells | 10–1000 µg/mL for 48 h | -Inhibit the canonical Wnt signaling pathway. | [166] |
| In vivo | ||||
| Freeze-dried strawberry | AOM/DSS induced male Crj: CD-1 mice | 2.5%, 5.0% or 10.0% for 20 weeks | -Reduce proinflammatory mediators and oncogenic signaling pathways. | [167] |
| Bilberry | ||||
| In vitro | ||||
| Ethanol extracts | HCT-116 cells | 4 mg/mL for 24 or 48 h | -Inhibit cancer cell proliferation. | [168] |
| Anthocyanin-rich extracts | HT-29 cell | 25–75 μg/mL (equivalents as cyanidin 3 glucoside) for 48 h | -Inhibit cell proliferation | [153] |
| HT-29 cells | 0–60 mg/mL for 24 h | -Inhibit cancer cell proliferation. -Induce apoptosis. | [168] | |
| HT-29 cells | 50–500 µg/mL for 72 h | -Suppress the DNA-damaging properties. | [155] | |
| HT-29 cells | 5–500 µg/mL for 1 to 24 h | -Exhibit cytotoxicity. -Decrease DNA damage and ROS level. | [169] | |
| Anthocyanin-rich extracts | HT-29 and NCM460 cells | 10–75 µg of monomeric anthocyanin/mL for 24–72 h | -Inhibit cancer cell proliferation. | [154] |
| Anthocyanin-rich extracts | Caco-2 cells | 0.1–100 nM for 1 h | -Exert potent intracellular antioxidant activity. | [143] |
| In vivo | ||||
| Anthocyanin-rich extracts | AOM treated Fischer 344 male rats | 3.85 g of monomeric anthocyanin/kg for 14 weeks | -Decrease the number of total and large ACF. | [158] |
| Mirtoselect and cyanidin-3-glucoside | Apc Min/+ mouse | 0.03%–0.3% for 12 weeks | -Decrease the total numbers of intestine adenomas. | [170] |
| Freeze-dried bilberry | Apc Min/+ mouse | 1564 mg/kg for 10 weeks | -Inhibit the formation of intestinal adenoma. | [171] |
| Cranberry | ||||
| In vitro | ||||
| Cranberry presscake and whole cranberry extract | HT-29 cells | 0–600 mg/mL for 4 days | -Exhibit antiproliferative activity. | [172] |
| Cranberry extracts and polyphenol fraction | HCT-116, SW480 and SW620 cells | 50–200 μg/mL (extract) and 6.5–78.8 μg/mL (fractions) for 48 h | -Enhance antiproliferative activity. | [173] |
| In vivo | ||||
| Cranberry extracts and dried cranberry | DSS induced murine colitis | 0.1% creanberry extract and 1.5% dry cranberry for 1 week | -Prevent colitis. -Decrease inflammatory cytokines. | [174] |
| Cranberry products | AOM induced Fisher 344 male | 50 g/kg for 17 weeks | -Inhibit colonic ACF formation. | [150] |
| Fr6 and purified proanthocyanidin | Xenografts Balb/c mice | 100 mg/kg proanthocyanidin and 250 mg/kg Fr6 for every 2 days for 3 weeks | -Decrease tumor growth and volume. | [175] |
| Juice of high-bush cranberry | DMH treated mouse | 65% gilaburu pulp and 45% water (pH: 3.09) for 30 weeks | -Inhibit tumor lesion at the initiation stage. | [176] |
| Mangosteen | ||||
| In vitro | ||||
| α-Mangostin and other xanthones extracts | HCT-116 cells | 2.5–30 μg/mL for 48 h | -Induce cytotoxicity and apoptosis. | [71] |
| α-Mangostin | HCT-116 cells | 14.8–25.6 µM for 24 h | -Inhibit proliferation. -Induce apoptosis and arrest cell cycle. | [177] |
| DLD-1 cells | 0 to 20 µM for 24 h | -Inhibit proliferation. -Induce apoptosis. | [178] | |
| HT-29 cells | 6–12 μM for 24 h | -Exert anti-proliferative activity. -Decrease Bcl-2 and β-catenin expresion. | [179] | |
| γ-Mangostin | HT-29 cells | 10–200 μM for 24 h | -Inhibits cancer cell proliferation. -Induces apoptosis and increases ROS. | [180] |
| In vivo | ||||
| Extracts of mangosteen pericarp | Established subcutaneous tumor of HCT-116 cells in NCR nude mice | 0.25% and 0.5% for 20 days | -Inhibit tumor growth and fewer blood vessels in tumor. | [181] |
| α-Mangostin | HT-29 colon cell xenogrft Balb/c nu/nu mice | 900 mg /kg for 2 or 4 weeks | -Decrease tumor masses and anti-apoptotic protein, Bcl-2, and β-catenin. | [179] |
| Her2/CT26 colon cell xenografts mice | 20 mg/kg daily for 3 days | -Reduce tumor growth by autophagy activation. | [182] | |
| DMH induce Fisher 344 rats | 0.02% and 0.05% for 5 weeks | -Inhibit development of ACF. -Decreases dysplastic foci, β-catenin accumulated crypts and lower PCNA. | [183] | |
| Crude methanolic extract | Mice were implanted with NL-17 cells | 0–250 mg/kg for 14 days | -Increase life span by decreasing tumor growth. | [184] |
| Blackberry | ||||
| In vitro | ||||
| Blackberry extract | HT-29 and HCT-116 cells | 25–200 µg/mL for 24 to 48 h | -Exert antiproliferative effects. | [145] |
| Anthocyanin-rich extracts from hull and crude blackberry | HT-29 cells | 13.6 to 49.2 µg of monomeric anthocyanins/mL for 48 to 72 h | -Induce significant antioxidant and antiproliferative activity. | [185] |
| Anthocyanin-rich extracts from crude blackberry | Caco-2 cells | 0.8, 1.6, 3.1, 6.3, 12.5 and 25 µg/mL for 24 h | -Inhibit peroxyl radical induced apoptosis. | [186] |
| In vivo | ||||
| Blackberry products | AOM induced Fisher 344 male | 50 g/kg for 17 weeks | -Inhibit colonic ACF formation. | [150] |
| Blackcurrant | ||||
| In vitro | ||||
| Black currant press residue extracts | Caco-2, HT-29, and HCT-116 cells | 0–125 μg GAE/mL for 24 to 48 h | -Suppress cancer cell proliferation. | [187] |
| Black currant extracts | HT-29 cells | 0.025% to 0.5% dry wt for 24 h | -Exert antiproliferative effect. | [142] |
| Methanol extracts of blackcurren | HT-29 cells | 0–60 mg/mL for 24 h | -Diminish cell proliferation via the p21WAF1 pathway. | [168] |
| IVD digestion and fecal fermentation | HT-29 and HT-115 cells | 0–50 µg/mL GAE for 24 h | -Exert anti-genotoxic, anti-mutagenic and anti-invasive activity. | [116] |
| Chokeberry | ||||
| In vitro | ||||
| In vitro gastric and pancreatic digestion of chokeberry juice | Caco-2 cells | 0 to 800 µM for 2 h a day for 4 days period | -Inhibit cell proliferation. -Arrest cell cycle at G2/M phase -Upregulate tumor suppressor CEACAM1 gene expresion. | [125] |
| Anthocyanin-rich extracts | HT-29 cells | 0 to 200 μg/mL for 48 h | -Suppress cancer cell proliferation. | [153] |
| HT-29 cells | 10–75 µg of monomeric anthocyanin/mL for 24–72 h | -Inhibit cancer cell proliferation. | [154] | |
| HT-29 cells | 50 μg monomeric anthocyanin/mL for 24 h | -Inhibit cell proliferation. -Block the cell cycle and increase cell cycle regulatory protein. | [188] | |
| In vivo | ||||
| Anthocyanin-rich extracts | AOM treated Fischer 344 male rats | 3.85 g of monomeric anthocyanin/kg for 14 weeks | -Inhibit colonic ACF formation. | [158] |
| Cloudberry | ||||
| In vitro | ||||
| Polyphenol-rich extracts | Caco-2 cells | 25, 50, and 75 μg of GAE/mL | -Inhibit cancer cell proliferation. | [162] |
| Methanolic extraction | HT-29 cells | 0–60 mg/mL for 24 to 48 h | -Disrupt cell proliferation. -Increases p21WAF1pathway. | [168] |
| In vivo | ||||
| Freeze dried cloubberry | Apc Min/+ mouse | 1564 mg/kg for 10 weeks | -Inhibits the formation of intestinal adenoma. | [171] |
| Seabuckthorn | ||||
| In vitro | ||||
| Polyphenol rich extracts | HT-29 cells | 0.025%–0.5% dry wt for 24 h | -Inhibit cancer cell proliferation. | [142] |
| Isorhamnetin | HT-29, HCT-116 and SW480 cells | 0–80 μmol/L for 3 days | -Decreases cancer cell proliferation. -Inhibits signaling pathway and arrests cell cycle. | [189] |
| In vivo | ||||
| Seabuckthorn seed oil | PhIP exposure Wistar rats | 2 to 8 mL/kg body wt for 12 to 36 h | -Improves oxidative stress and decreases abnormal cancer related gene expression. | [190] |
| Lingonberry | ||||
| In vitro | ||||
| Polyphenol-rich extracts | Caco-2 cells | 25, 50, and 75 μg of GAE/mL | -Induce antiproliferative activity. | [162] |
| Anthocyanin-rich extract | HT-29 cells | 0.025%–0.5% dry wt for 24 h | -Suppress the growth of cancer cells. | [142] |
| HT-29 cells | 0–60 mg/mL for 24 to 48 h | -Decrease cell proliferation proliferation via p21WAF1pathway. | [168] | |
| In vivo | ||||
| Freeze dried lingonberry | Apc Min/+ mouse | 1564 mg/kg for 10 weeks | -Decrease adenoma formation. | [171] |
| Barberry | ||||
| In vitro | ||||
| Berberine | SW480 cells | 5–50 µM for 12–72 h | -Suppresses cells growth. -Arrests cell cycle. -Induces apoptosis. -Inhibits angiogenesis and inflammation markers. | [191] |
| Acai Berry | ||||
| In vitro | ||||
| Polyphenolic extracts | SW480, HT-29 and CCD-18Co cells | 5–20 mg/L for 48 h | -Suppress cells growth. -Show anti-inflammatory activity. | [192] |
| In vivo | ||||
| Spray-dried acai powder | DMH in male Wistar rats | 2.5% or 5.0% acai power for 20 weeks | -Reduces the number of aberrant crypts, invasive tumors and tumor multiplicity. | [193] |
| Goji berry | ||||
| In vitro | ||||
| Lycium barbarum polysaccharides | SW480 and Caco-2 cells | 100–1000 mg/L for 1, 2, 3, 4,or 5 days | -Decreases cells growth by intrupting cell cycle. | [194] |
| Silverberry | ||||
| In vitro | ||||
| Extracts from seed and flesh of cherry silverberry | HT-29 cells | Seed extract (100–1600 g/mL) and flesh extract (200–3200 g/mL) for 48 h | -Exert anti-inflammation and anti-proliferation activities. | [195] |
| White currant | ||||
| In vivo | ||||
| Freeze dried white currant | Min mice | 10% for 10 weeks | -Prevents cancer initiation and progression. | [196] |
| Arctic bramble | ||||
| In vitro | ||||
| Polyphenol-rich extracts | Caco 2 cells | 25, 50, and 75 μg of GAE/mL | -Reduce cancer cell proliferation. | [162] |
| Elderberry | ||||
| In vitro | ||||
| Anthocyanin-rich extracts | HT-29 cells | 0 to 200 μg/mL for 48 h | -Inhibit cancer cell proliferation. | [153] |
| Jamun berry | ||||
| In vitro | ||||
| ETs rich jamun berry extracts | Human 293T cells | 10–1000 µg/mL for 48 h | -Exert chemopreventive activity. -Inhibit the canonical Wntsignaling pathway. | [166] |
| Rosehip | ||||
| In vitro | ||||
| Polyphenol rich extracts | HT-29 cells | 0.025,0.05, 0.25, and 0.5% dry wt for 24 h | -Inhibit cancer cell proliferation. | [142] |
| 62.5–1000 g/L for 24 h | -Suppress cancer cell growth. | [93] | ||
| Emblic | ||||
| In vitro | ||||
| Ethanolic extracts | HT-29 cells | 10-100 μg/mL for 48 h | -Inhibit cancer cell proliferation. | [197] |
| Water extract | COLO320 cells | 0, 20, 40, 80, or 160 μg/mL PE for 24, 48, 72, or 96 h | -Suppress necrosis and delays mitotic progression. -Induce apoptosis. | [198] |
4.2. Blueberry
4.3. Grape
4.4. Strawberry
4.5. Bilberry
4.6. Cranberry
4.7. Mangosteen
4.8. Blackberry
4.9. Blackcurrant
4.10. Chokeberry
4.11. Cloudberry
4.12. Seabuckthorn
4.13. Lingonberry
4.14. Barberry
4.15. Açai Berry
4.16. Gogi Berry
4.17. Silverberry
4.18. White Currant
4.19. Arctic Bramble
4.20. Elderberry
4.21. Jamun Berry
4.22. Rosehip
4.23. Emblic
5. Biological Activities of Berries Against colon Cancer: Human Studies
| Fresh or Processed Berry | Study Subjects | Duration and Dose/Intervention | Key/Major Findings | References |
|---|---|---|---|---|
| Anthocyanin-rich standardized bilberry extract, mirtocyan | 25 colorectal cancer patients | 0.5–2.0 g/day for 7 days before surgery |
| [216] |
| Black raspberry power | 20 colorectral cancer patients | 60 g/day of black raspberry orally for 1 to 9 weeks |
| [217] |
| 24 colorectral cancer patients | 20 g in 100 mL drinking water, 3 times/day for 1–9 weeks |
| [218] | |
| Black raspberry | 14 patients with FAP | Oral treatment containing 60 g black raspberr/day, and suppositories containing 720 mg black raspberry/day for 9 months. |
| [219] |
| FL and CAM30 prepared from blackcurrant extract | 30 healthy volunteers (Aged 20–60 years) | Both product contain 672 mg blackcurrant power |
| [220] |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Tenesa, A.; Dunlop, M.G. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat. Rev. Genet. 2009, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Ansary-Moghaddam, A.; Clifton, P.; Czernichow, S.; Parr, C.L.; Woodward, M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int. J. Cancer 2009, 125, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.M.; Collins, P.D. Colon cancer: A civilization disorder. Dig. Dis. 2011, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; Benson, A.B.; Fuchs, C.S. Impact of diabetes mellitus on outcomes in patients with colon cancer. J. Clin. Oncol. 2003, 21, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.J.; Wickramasinghe, N.S.; Ivanova, M.M.; Isaacs, S.M.; Dougherty, S.M.; Imbert-Fernandez, Y.; Cunningham, A.R.; Chen, C.; Klinge, C.M. Anacardic acid inhibits estrogen receptor α–DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol. Cancer Ther. 2010, 9, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Houlston, R.S. A systematic review and meta-analysis of familial colorectal cancer risk. Am. J. Gastroenterol. 2001, 96, 2992–3003. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Boland, C.R. Hereditary nonpolyposis colorectal cancer: The syndrome, the genes, and historical perspectives. J. Natl. Cancer Inst. 1995, 87, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Dietary phenolics against colorectal cancer—From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Smits, A.M.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.R.; Rowan, A.; Smith, M.E.; Kerr, I.B.; Bodmer, W.F.; Gannon, J.V.; Lane, D.P. P53 mutations in colorectal cancer. Proc. Natl. Acad. Sci. USA 1990, 87, 7555–7559. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Albertsen, H.; Herrick, J.; Levin, T.R.; Sweeney, C.; Murtaugh, M.A.; Wolff, R.K.; Slattery, M.L. Evaluation of a large, population-based sample supports a cpg island methylator phenotype in colon cancer. Gastroenterology 2005, 129, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Irahara, N.; Shima, K.; Kure, S.; Kirkner, G.J.; Schernhammer, E.S.; Hazra, A.; Hunter, D.J.; Quackenbush, J.; Spiegelman, D. Comprehensive biostatistical analysis of cpg island methylator phenotype in colorectal cancer using a large population-based sample. PLoS ONE 2008, 3, e3698. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin. Colorectal Cancer 2003, 2, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J.; Balmain, A. Tgf-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Takaku, K.; Oshima, M.; Miyoshi, H.; Matsui, M.; Seldin, M.F.; Taketo, M.M. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and apc genes. Cell 1998, 92, 645–656. [Google Scholar] [CrossRef]
- Eppert, K.; Scherer, S.W.; Ozcelik, H.; Pirone, R.; Hoodless, P.; Kim, H.; Tsui, L.C.; Bapat, B.; Gallinger, S.; Andrulis, I.L. MADR2 maps to 18q21 and encodes a TGFβ–regulated MAD–related protein that is functionally mutated in colorectal carcinoma. Cell 1996, 86, 543–552. [Google Scholar] [CrossRef]
- Kojima, M.; Morisaki, T.; Sasaki, N.; Nakano, K.; Mibu, R.; Tanaka, M.; Katano, M. Increased nuclear factor-kb activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004, 24, 675–682. [Google Scholar] [PubMed]
- Schmoll, H.J.; van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Mendel, J. Evidenced based medicine. Benefits, limitations and issues for complementary and alternative medicine. Aust. J. Holist. Nurs. 2004, 11, 21–29. [Google Scholar] [PubMed]
- Jaganathan, S.K.; Mandal, M. Honey constituents and its apoptotic effect in colon cancer cells. J. ApiProduct ApiMed. Sci. 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Kleihues, P.; Stewart, B.W. World Cancer Report; IARC Press: Lyon, France, 2003; Volume 57. [Google Scholar]
- Bishayee, A.; Haskell, Y.; Do, C.; Siveen, K.S.; Mohandas, N.; Sethi, G.; Stoner, G.D. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: Preclinical and clinical evidence. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Derry, M.M.; Raina, K.; Agarwal, C.; Agarwal, R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009, 67, S145–S150. [Google Scholar] [CrossRef] [PubMed]
- Hautsalo, J.; Vestberg, M.; Parikka, P.I.; Kukkonen, S.; Karhu, S.; Tahvonen, R. Biological control of strawberry crown rot is substrate dependent phenomenon. J. Berry Res. 2016. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Polyphenol compounds and other quality traits in blueberry cultivars. J. Berry Res. 2015, 5, 117–130. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Pissard, A.; Lateur, M.; Baeten, V.; Magein, H.; Dupont, P.; Tabart, J.; Pincemail, J.; Kevers, C. Determination of total phenolic compound content and antioxidant activity in cherry species and cultivars. J. Berry Res. 2016. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanit. 2007, 43, 348–361. [Google Scholar]
- Mazur, W.M.; Uehara, M.; Wähälä, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar] [PubMed]
- Clifford, M.N. Anthocyanins–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Clifford, M.N.; Scalbert, A. Ellagitannins, occurrence in food, bioavailability and cancer prevention. J. Food Sci. Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Block, G.; James, L.D. Folate intake and food sources in the US population. Am. J. Clin. Nutr. 1989, 50, 508–516. [Google Scholar] [PubMed]
- Ivanovska, N.; Philipov, S. Study on the anti-inflammatory action of berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996, 18, 553–561. [Google Scholar] [CrossRef]
- Ovaskainen, M.L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [PubMed]
- Akao, Y.; Nakagawa, Y.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Hilz, H.; Bakx, E.J.; Schols, H.A.; Voragen, A.G.J. Cell wall polysaccharides in black currants and bilberries-characterisation in berries, juice, and press cake. Carbohydr. Polym. 2005, 59, 477–488. [Google Scholar] [CrossRef]
- Cocco, C.; Magnani, S.; Luigia Maltoni, M.; Quacquarelli, I.; Cacchi, M.; Eduardo Corrêa Antunes, L.; Filippo D’Antuono, L.; Faedi, W.; Baruzzi, G. Effects of site and genotype on strawberry fruits quality traits and bioactive compounds. J. Berry Res. 2015, 5, 145–155. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Liguori, G.; Vonella, V.; Zappia, R.; Inglese, P. Evaluation of fruit quality and antioxidant activity of kiwifruit during ripening and after storage. J. Berry Res. 2016. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, J.; Mazzoni, L.; Balducci, F.; Cappelletti, R.; Capocasa, F.; Battino, M.; Dobson, G.; Stewart, D.; Mezzetti, B. Use of wild genotypes in breeding program increases strawberry fruit sensorial and nutritional quality. J. Agric. Food Chem. 2014, 62, 3944–3953. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.; Bonat Celli, G.; Ghanem, A.; Brooks, M.S.L. Effect of frozen storage on polyphenol content and antioxidant activity of haskap berries (Lonicera caerulea L.). J. Berry Res. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- López-Aranda, J.M.; Gómez, F.; Puga, M.; Zamora, R.; Daugovish, O.; Cotero, M.A. Chemical soil fumigation for raspberry nursery in Jalisco (Mexico). J. Berry Res. 2016. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Seeram, N.P. Bioactive Polyphenols from Foods and Dietary Supplements: Challenges and Opportunities; ACS Symp. Ser. Oxford University Press: Oxford, UK, 2006; pp. 25–38. [Google Scholar]
- Probst, Y. A review of the nutrient composition of selected Rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, Y.Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; L. Quiles, J.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y.; Chen, P. Resveratrol content in strawberry fruit is affected by preharvest conditions. J. Agric. Food Chem. 2007, 55, 8269–8274. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [PubMed]
- Cocetta, G.; Karppinen, K.; Suokas, M.; Hohtola, A.; Häggman, H.; Spinardi, A.; Mignani, I.; Jaakola, L. Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. J. Plant Physiol. 2012, 169, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.L.; Bomser, J.; Smith, M.A.L.; Singletary, K. Isolation of bioactive constituents from vaccinium myrtillus (bilberry) fruits and cell cultures. Plant Sci. 1998, 131, 95–103. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Fu, C.; Loo, A.E.K.; Chia, F.P.P.; Huang, D. Oligomeric proanthocyanidins from mangosteen pericarps. J. Agric. Food Chem. 2007, 55, 7689–7694. [Google Scholar] [CrossRef] [PubMed]
- Haruenkit, R.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Sajewicz, M.; Kowalska, T.; Delgado-Licon, E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Trakhtenberg, S. Comparative study of health properties and nutritional value of durian, mangosteen, and snake fruit: Experiments in vitro and in vivo. J. Agric. Food Chem. 2007, 55, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, J.; Mulero, J.; Marhuenda, J.; Cerdá, B.; Avilés, F. Changes in antioxidant enzymes in metabolic syndrome patients after consumption a citrus-based juice enriched with Aronia melanocarpa. J. Nutr. Disord. Ther. 2015, 5. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. The action of berry phenolics against human intestinal pathogens. Biofactors 2005, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, M.; Korpelainen, V.; Hoppula, K.; Virtanen, V. Chemical composition of ripe fruits of Rubus chamaemorus L. Grown in different habitats. J. Sci. Food Agric. 2012, 92, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z. Chemical and nutritional constituents of sea buckthorn juice. Pak. J. Nutr. 2004, 3, 99–106. [Google Scholar]
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.H.; Minihane, A.M. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, H.M.; Lindstedt, A.; Järvinen, R.; Sinkkonen, J.; Graça, G.; Viitanen, M.; Kallio, H.; Gil, A.M. 1H-NMR-based metabolic fingerprinting of urine metabolites after consumption of lingonberries (Vaccinium vitis-idaea) with a high-fat meal. Food chem. 2013, 138, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.; Crozier, A.; Gill, C.I.R. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: Potential impact on colonic health. BioFactors 2014, 40, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Plant genus elaeagnus: Underutilized lycopene and linoleic acid reserve with permaculture potential. Fruits 2015, 70, 191–199. [Google Scholar] [CrossRef]
- Määttä, K.; Kamal-Eldin, A.; Törrönen, R. Phenolic compounds in berries of black, red, green, and white currants (Ribes sp.). Antioxid. Redox Signal. 2001, 3, 981–993. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. New beverages of lemon juice with elderberry and grape concentrates as a source of bioactive compounds. J. Food Sci. 2012, 77, C727–C733. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.F.; Marques, M.C.; Mercadante, A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011, 126, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Tumbas, V.T.; Čanadanović-Brunet, J.M.; Četojević-Simin, D.D.; Ćetković, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Papasian, C.; Hentges, S.; Banerjee, S.; Haque, I.; Banerjee, S.K. Emblica officinalis extract induces autophagy and inhibits human ovarian cancer cell proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS ONE 2013, 8, e72748. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.Y.; Yu, X.Y.; Liu, D.; Wan, H.X. Evaluation of cellular antioxidant and antiproliferative activities of five main Phyllanthus emblica L. Cultivars in China. Indian J. Pharm. Sci. 2015, 77, 274–282. [Google Scholar] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, I.; Hjerkinn, E.M.; Seljeflot, I.; Arnesen, H.; Tonstad, S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br. J. Nutr. 2008, 99, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Dietary flavonoids as antioxidants. Forum Nutr. 2009, 61, 87–91. [Google Scholar] [PubMed]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristic, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Alvarez-Suarez, J.M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Giampieri, F. A pilot study of the photoprotective effects of strawberry-based cosmetic formulations on human dermal fibroblasts. Int. J. Mol. Sci. 2015, 16, 17870–17884. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magnuson, B.A.; Giusti, M.M. Analysis of anthocyanins in rat intestinal contents impact of anthocyanin chemical structure on fecal excretion. J. Agric. Food Chem. 2005, 53, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pittman, H.E.; McKay, S.; Prior, R.L. Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs. J. Nutr. 2005, 135, 2417–2424. [Google Scholar] [PubMed]
- Kida, K.; Suzuki, M.; Matsumoto, N.; Nanjo, F.; Hara, Y. Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J. Agric. Food Chem. 2000, 48, 4151–4155. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Kraus, M.; Scheppach, W.; Ackermann, M.; Ridder, F.; Richling, E. Studies on apple and blueberry fruit constituents: Do the polyphenols reach the colon after ingestion? Mol. Nutr. Food. Res. 2006, 50, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.I.R.; McDougall, G.J.; Glidewell, S.; Stewart, D.; Shen, Q.; Tuohy, K.; Dobbin, A.; Boyd, A.; Brown, E.; Haldar, S. Profiling of phenols in human fecal water after raspberry supplementation. J. Agric. Food Chem. 2010, 58, 10389–10395. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Brown, E.M.; Latimer, C.; Allsopp, P.; Ternan, N.G.; McMullan, G.; McDougall, G.J.; Stewart, D.; Crozier, A.; Rowland, I.; Gill, C.I.R. In vitro and in vivo models of colorectal cancer: Antigenotoxic activity of berries. J. Agric. Food Chem. 2014, 62, 3852–3866. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; McDougall, G.J.; Stewart, D.; Pereira-Caro, G.; González-Barri, R.; Allsopp, P.; Magee, P.; Crozier, A.; Rowland, I.; Gill, C.I.R. Persistence of anticancer activity in berry extracts after simulated gastrointestinal digestion and colonic fermentation. PLoS ONE 2012, 7, e49740. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavéra, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro-and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [PubMed]
- Appeldoorn, M.M.; Vincken, J.-P.; Aura, A.-M.; Hollman, P.C.H.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl) acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Durgo, K.; Belščak-Cvitanović, A.; Stančić, A.; Franekić, J.; Komes, D. The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon adenocarcinoma. J. Med. Food 2012, 15, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Kuo, C.T.; Cho, S.J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Weng, Y.I.; Huang, T.H.M.; Tichelaar, J.; Yearsley, M. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.M.; Popa, G.; Gill, C.I.R.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, Y.J.; Shin, H.K.; Park, J.H.Y. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition 2005, 21, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- God, J.; Tate, P.L.; Larcom, L.L. Red raspberries have antioxidant effects that play a minor role in the killing of stomach and colon cancer cells. Nutr. Res. 2010, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Bomser, J.A.; Scheerens, J.C.; Giusti, M.M. Effect of black raspberry (Rubus occidentalis L.) extract variation conditioned by cultivar, production site, and fruit maturity stage on colon cancer cell proliferation. J. Agric. Food Chem. 2011, 59, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Kuo, C.T.; Huang, T.H.M.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.W. Black raspberries protectively regulate methylation of wnt pathway genes in precancerous colon tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Fang, W.; Wang, L.S.; Stoner, G.D.; Yang, W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/− and muc2−/− mouse models of colorectal cancer. Cancer Prev. Res. 2010, 3, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Montrose, D.C.; Horelik, N.A.; Madigan, J.P.; Stoner, G.D.; Wang, L.S.; Bruno, R.S.; Park, H.J.; Giardina, C.; Rosenberg, D.W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2011, 32, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Kuo, C.T.; Stoner, K.; Yearsley, M.; Oshima, K.; Yu, J.; Huang, T.H.M.; Rosenberg, D.; Peiffer, D.; Stoner, G. Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis. Carcinogenesis 2013, 34, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, Ã.K.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.Y.; Zhang, Z.Y.; Zhang, X.W.; Yoshioka, M.; Yang, Y.N.; Li, J. Anthocyanins extracted from chinese blueberry (Vaccinium uliginosum L.) and its anticancer effects on DLD-1 and COLO205 cells. Chin. Med. J. 2010, 123, 2714–2719. [Google Scholar] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Afaq, F.; Khan, N.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. 2009, 48, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Akoh, C.C.; Fischer, J.; Krewer, G. Effect of anthocyanin fractions from selected cultivars of georgia-grown blueberries on apoptosis and phase ii enzymes. J. Agric. Food Chem. 2007, 55, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Verghese, M.; Shackelford, L.; Walker, L.T.; Khatiwada, J.; Ogutu, S.; Williams, D.S.; Jones, J.; Guyton, M.; Asiamah, D. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, Å.; Bränning, C.; Molin, G.; Adawi, D.; Hagslätt, M.L.; Jeppsson, B.; Nyman, M.; Ahrne, S. Blueberry husks and probiotics attenuate colorectal inflammation and oncogenesis, and liver injuries in rats exposed to cycling dss-treatment. PLoS ONE 2012, 7, e33510. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, A.M.; Mattheyse, M.; Ellis, B.; Loos, B.; Thomas, M.; Smith, R.; Peters, S.; Smith, C.; Myburgh, K. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007, 258, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M.N. Structure–function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Esselen, M.; Fritz, J.; Hutter, M.; Teller, N.; Baechler, S.; Boettler, U.; Marczylo, T.H.; Gescher, A.J.; Marko, D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55, S143–S153. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Patil, B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis. Food Chem. Toxicol. 2011, 49, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Lazzè, M.C.; Pizzala, R.; Gutiérrez Pecharromán, F.J.; Gatòn Garnica, P.; Antolín Rodríguez, J.M.; Fabris, N.; Bianchi, L. Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J. Med. Food 2009, 12, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Caderni, G.; Vignali, F.; Salvadori, M.; Giannini, A.; Biggeri, A.; Gee, J.; Przybylska, K.; Cheynier, V.; Dolara, P. Effect of polyphenolic extracts from red wine and 4–OH–coumaric acid on 1, 2–dimethylhydrazine–induced colon carcinogenesis in rats. Eur. J. Nutr. 2005, 44, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, D.; Vinardell, M.P.; Noé, V.; van Delft, J.H.; Alcarraz-Vizán, G.; van Breda, S.G.; Staal, Y.; Günther, U.L.; Reed, M.A.; Torres, J.L. A lyophilized red grape pomace containing proanthocyanidin-rich dietary fiber induces genetic and metabolic alterations in colon mucosa of female C57BL/6J mice. J. Nutr. 2011, 141, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, S.Y.; Yin, J.J.; Parry, J.; Yu, L.L. Enhancing antioxidant, antiproliferation, and free radical scavenging activities in strawberries with essential oils. J. Agric. Food Chem. 2007, 55, 6527–6532. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.H.Y. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin a, on Wnt signaling. J. Agric. Food Chem. 2009, 58, 3965–3969. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in crj: Cd-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.K.; Koponen, J.M.; Mykkänen, H.M.; Törrönen, R. Berry phenolic extracts modulate the expression of p21WAF1 and bax but not Bcl-2 in HT-29 colon cancer cells. J. Agric. Food Chem. 2007, 55, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidative efficiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar]
- Cooke, D.; Schwarz, M.; Boocock, D.; Winterhalter, P.; Steward, W.P.; Gescher, A.J.; Marczylo, T.H. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the apcmin mouse model of intestinal carcinogenesis—Relationship with tissue anthocyanin levels. Int. J. Cancer 2006, 119, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, M.; Pajari, A.M.; Paivarinta, E.; Misikangas, M.; Rajakangas, J.; Marttinen, M.; Oikarinen, S. Berries as chemopreventive dietary constituents-a mechanistic approach with the apcmin/+ mouse. Asia Pac. J. Clin. Nutr. 2008, 17, 123–125. [Google Scholar] [PubMed]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr. 2004, 134, 1529–1535. [Google Scholar] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Kim, J.; Sun, Q.; Kim, D.; Park, C.S.; Lu, T.S.; Park, Y. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem. 2015, 167, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.J.; Kurowska, E.M.; Freeman, D.J.; Chambers, A.F.; Koropatnick, J. In vivo inhibition of growth of human tumor lines by flavonoid fractions from cranberry extract. Nutr. Cancer 2006, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ulger, H.; Ertekin, T.; Karaca, O.; Canoz, O.; Nisari, M.; Unur, E.; Elmali, F. Influence of gilaburu (Viburnum opulus) juice on 1,2-dimethylhydrazine (DMH)-induced colon cancer. Toxicol. Ind. Health 2013, 29, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Kuriyama, I.; Nakahara, T.; Kawashima, Y.; Yoshida, H. Inhibitory effects of α-mangostin on mammalian DNA polymerase, topoisomerase, and human cancer cell proliferation. Food Chem. Toxicol. 2013, 59, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Iinuma, M.; Naoe, T.; Nozawa, Y.; Akao, Y. Characterized mechanism of α-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer dld-1 cells. Bioorg. Med. Chem. 2007, 15, 5620–5628. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Thomas-Ahner, J.M.; Li, J.; Riedl, K.M.; Nontakham, J.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol. Nutr. Food Res. 2013, 57, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.F.; Yang, L.L. Gamma-mangostin, a micronutrient of mangosteen fruit, induces apoptosis in human colon cancer cells. Molecules 2012, 17, 8010–8021. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.A.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. In vitro and in vivo anti-colon cancer effects of garcinia mangostana xanthones extract. BMC Complement. Altern. Med. 2012, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hong, E.H.; Lee, B.R.; Park, M.H.; Kim, J.W.; Pyun, A.R.; Kim, Y.J.; Chang, S.Y.; Chin, Y.W.; Ko, H.J. Α-mangostin reduced ER stress-mediated tumor growth through autophagy activation. Immune Netw. 2012, 12, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Nabandith, V.; Suzui, M.; Morioka, T.; Kaneshiro, T.; Kinjo, T.; Matsumoto, K.; Akao, Y.; Iinuma, M.; Yoshimi, N. Inhibitory effects of crude alpha-mangostin, a xanthone derivative, on two different categorie of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in the rat. Asian Pac. J. Cancer Prev. 2004, 5, 433–438. [Google Scholar] [PubMed]
- Kosem, N.; Ichikawa, K.; Utsumi, H.; Moongkarndi, P. In vivo toxicity and antitumor activity of mangosteen extract. J. Nat. Med. 2013, 67, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food. 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Holtung, L.; Grimmer, S.; Aaby, K. Effect of processing of black currant press-residue on polyphenol composition and cell proliferation. J. Agric. Food Chem. 2011, 59, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. Induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.; Chen, C.; Cai, S.; Hu, J. Isorhamnetin suppresses colon cancer cell growth through the PI3K-Akt-mTOR pathway. Mol. Med. Rep. 2014, 9, 935–940. [Google Scholar] [PubMed]
- Li, R.J.; Tian, J.J.; Li, W.Q.; Cheng, F.Q.; Gao, G.S. Effect of 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine on oxidative stress and gene expression of c-fos, c-jun, p16 and Rb in rat colons and protective role of seabuckthorn seed oil. J. Environ. Sci. Health B 2014, 49, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Patil, B.S. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur. J. Pharmacol. 2012, 688, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.M.D.S.; Noratto, G.; Martino, H.S.D.; Arbizu, S.; Peluzio, M.D.C.G.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Pro-apoptotic activities of polyphenolics from açai (Euterpe oleracea Martius) in human SW-480 colon cancer cells. Nutr. Cancer 2014, 66, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.F.; Romualdo, G.R.; Ribeiro, D.A.; Barbisan, L.F. Acai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem. Toxicol. 2013, 58, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Xiao, B.; Jiang, Z.; Zhao, J.; Huang, X.; Guo, J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med. Oncol. 2011, 28, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, Y.K.; Park, O.J. Cherry silver berry (Elaeagnus multiflora) extracts exert antiinflammatory effects by inhibiting COX-2 and akt signals in HT-29 colon cancer cells. Food Sci. Biotechnol. 2010, 19, 1673–1677. [Google Scholar] [CrossRef]
- Rajakangas, J.; Misikangas, M.; Päivärinta, E.; Mutanen, M. Chemoprevention by white currant is mediated by the reduction of nuclear β-catenin and NF-κB levels in min mice adenomas. Eur. J. Nutr. 2008, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, D. Antioxidant and antitumor activity of Phyllanthus emblica in colon cancer cell lines. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 189–195. [Google Scholar]
- Guo, X.; Ni, J.; Liu, X.; Xue, J.; Wang, X. Phyllanthus emblica L. Fruit extract induces chromosomal instability and suppresses necrosis in human colon cancer cells. Int. J. Vitam. Nutr. Res. 2013, 83, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale swartz) from colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of polyphenol-rich chokeberry juice on antioxidant/pro-oxidant status in healthy subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Berge, V. Cloudberry: An important source of ellagic acid, an anti-oxidant. Tidsskr. Nor. Laegeforen. 2003, 123, 1856–1857. [Google Scholar] [PubMed]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V. Antioxidant and Antiproliferative Activities of Fruits; Cornell University, Jan.: Ithaca, NY, USA, 2001. [Google Scholar]
- Awan, M.S.; Ali, S.; Ali, A.; Hussain, A.; Ali, M. A comparative study of barberry fruits in terms of its nutritive and medicinal contents from CKNP region, gilgit-baltistan, pakistan. J. Biodivers. Environ. Sci. 2014, 5, 9–17. [Google Scholar]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.C.; So, K.F. Use of anti-aging herbal medicine, lycium barbarum, against aging-associated diseases. What do we know so far? Cell. Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Chandra, D. Pharmacological potentials of Syzygium cumini: A review. J. Sci. Food Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L.L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; Marques, M.; Witcher, M. Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.M.; Riedl, K.; Schwartz, S.; Frankel, W. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.A.; Bobe, G.; Sardo, C.; Wang, L.S.; Kuo, C.T.; Stoner, G.; Colburn, N.H. Plasma cytokines as potential response indicators to dietary freeze-dried black raspberries in colorectal cancer patients. Nutr. Cancer 2012, 64, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.S.; Seguin, C.; Liu, P.; Huang, T.H.M.; Frankel, W.L.; Stoner, G.D. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. https://doi.org/10.3390/molecules21020169
Afrin S, Giampieri F, Gasparrini M, Forbes-Hernandez TY, Varela-López A, Quiles JL, Mezzetti B, Battino M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules. 2016; 21(2):169. https://doi.org/10.3390/molecules21020169
Chicago/Turabian StyleAfrin, Sadia, Francesca Giampieri, Massimiliano Gasparrini, Tamara Y. Forbes-Hernandez, Alfonso Varela-López, José L. Quiles, Bruno Mezzetti, and Maurizio Battino. 2016. "Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment" Molecules 21, no. 2: 169. https://doi.org/10.3390/molecules21020169